Polyurethanes
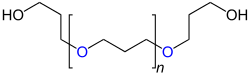
| General structure of polyurethanes |

|
| Repeat unit for linear polyurethanes that have been produced from a diol and diisocyanate. The urethane groups are marked in blue . R 1 stands for the “remainder” of the diol used for the synthesis (HO − R 1 −OH), R 2 for the “remainder” of the diisocyanate (OCN − R 2 −NCO). |
Polyurethanes ( abbreviation PUR ; in linguistic usage also PU ) are plastics or synthetic resins that result from the polyaddition reaction of dialcohols ( diols ) or polyols with polyisocyanates . The urethane group ( ) is characteristic of polyurethanes .
Diols and diisocyanates lead to linear polyurethanes ; crosslinked polyurethanes can be produced by reacting triisocyanate-diisocyanate mixtures with triol-diol mixtures. The properties of PU can be varied within a wide range. Depending on the degree of crosslinking and / or the isocyanate or OH component used, thermosets , thermoplastics or elastomers are obtained . Quantitatively, polyurethane foams , as soft or rigid foam is most important. However, polyurethanes are also used as molding compounds for compression molding , as casting resins (isocyanate resins), as ( textile ) elastic fiber materials, polyurethane paints and as polyurethane adhesives .
history
In 1937 a research group led by Otto Bayer synthesized polyurethanes for the first time from 1,4-butanediol and octane-1,8-diisocyanate and later from hexane-1,6-diisocyanate in the laboratories of the IG Farben plant in Leverkusen . The corresponding polyurethane was called Igamid U or Perlon U. Further tests showed that tolylene diisocyanate was significantly more reactive than 1,6-hexane diisocyanate and that reactions with triols led to three-dimensionally crosslinked polyurethanes. In 1940 industrial production began in Leverkusen. However, due to the Second World War and the associated scarcity of raw materials, the market for polyurethanes developed only very slowly at first. Therefore, until the end of World War II, polyurethanes were only used for military purposes in aircraft construction. In 1952, less than 100 t of the important polyisocyanate toluene diisocyanate (TDI) were available per year . From 1952 to 1954, polyester foams were developed, which further increased commercial interest in polyurethanes. With the use of polyether polyols, the importance of polyurethanes grew rapidly. The greater scope for variation in the production of polyether polyols has resulted in a considerable expansion of the applications. In 1960, over 45,000 t of foam were produced.
By 2002, global consumption had risen to around 9 million tons of polyurethane, and by 2007 it rose further to over 12 million tons. The annual growth rate is approx. 5%. In 2011, production in Germany alone, with the main producers Covestro and BASF, was just under 1 million tons, of which around 32% was for building insulation, 20% for furniture and mattresses, 14% for automotive engineering and 10% for paints and coatings.
properties
Polyurethanes can have different properties depending on the choice of polyisocyanate and polyol . The density of unfoamed polyurethane varies between around 1000 and 1250 kg / m³. Typical densities are around 5 to 40 kg / m³ for soft block foam or 30 to 90 kg / m³ for hard block foam.
- toxicity
Isocyanates can trigger allergies and are suspected of causing cancer . When polyurethanes have reacted completely and no longer contain any monomers, they generally no longer have any harmful properties. Furthermore, volatile additives can be added to the polyurethane, such as flame retardants or plasticizers , which can be absorbed dermally (skin) or inhalatively (breathing) depending on the use. Guidelines and information sheets for the safe handling of polyurethane raw materials can be obtained from the manufacturers or from ISOPA (European Association of Diisocyanate and Polyol Manufacturers).
Manufacturing
| Diisocyanate monomers (selection) |
 Hexamethylene-1,6-diisocyanate (HDI) |
 Toluene-2,4-diisocyanate (TDI) |
 Diphenylmethane 4,4'-diisocyanate (MDI) |
 Isophorone diisocyanate (IPDI) |
| common diol components |
| Polyether -Polyol: Oxygen atoms of the ether are marked in blue . |
| Polyester polyol made from adipic acid and 1,4-butanediol . Oxygen atoms and carbon atoms of the carboxylic acid ester groups are marked in blue . |
Polyurethanes result from the polyaddition reaction of polyisocyanates with polyhydric alcohols, the polyols . The linkage occurs through the reaction of an isocyanate group (-N = C = O) of one molecule with a hydroxyl group (-OH) of another molecule to form a urethane group (-NH-CO-O-). In contrast to polycondensation , no by-products are split off.
Only a few different isocyanate components are used:
- Hexamethylene diisocyanate (HDI)
- Toluene diisocyanate (TDI)
- Methylenedi (phenyl isocyanate) (MDI)
- Polymeric diphenylmethane diisocyanate (PMDI)
- Naphthylene diisocyanate (NDI)
- Isophorone diisocyanate (IPDI)
- 4,4'-diisocyanatodicyclohexylmethane (H12MDI)
Due to the high volatility and the consequent dangerous processing of the above monomers, in most cases only prepolymers are used by processors, which, however, always contain a residual monomer content. This is particularly the case with HDI. The usual residual monomer proportions in HDI trimer products (e.g. Desmodur N, Tolonate HDT, Basonat or Duranate) are <0.5% HDI and are therefore classified as non-toxic according to the manufacturer's classification and thus in the professional area, taking into account the manufacturer's protective instructions usable.
The later properties are essentially determined by the polyol component, because in order to achieve the desired properties it is usually not the isocyanate component that is adapted (chemically changed), but the polyol component. Mechanical properties can be influenced depending on the chain length and number of branches in the polyol. For example, the use of polyester polyols in addition to the more common polyether polyols leads to better stability, because polyester polyols have a higher melting point and thus solidify when the polyurethane is applied.
Polyurethane formation requires at least two different monomers, in the simplest case a diol and a diisocyanate. The polyreaction takes place in stages. First, a bifunctional molecule with an isocyanate group (-N = C = O) and a hydroxyl group (-OH) is formed from diol and diisocyanate . This can react with other monomers at both ends . This creates short chains of molecules, so-called oligomers . These can react with other monomers, other oligomers or polymers that have already been formed .
Networking
Linear polyurethanes can be crosslinked with an excess of diisocyanate. The addition of an isocyanate group to a urethane group forms an allophanate group.
By trimerizing three isocyanate groups, it is also possible to form an isocyanurate group. If multifunctional isocyanates are used, the highly branched polyisocyanurates (PIR) are formed, see there.
Alternatively, crosslinked or branched polyurethanes can also be produced by adding substances with more than two isocyanate groups, such as PMDI , and triols, such as glycerol . The use of multiple amines, such as ethylenediamine , also leads to crosslinking. The reaction of isocyanates with amines leads to urea groups.
These are still reactive and allow the addition of a further isocyanate group, forming a biuret group.
If a certain polyurethane is to be produced in practice, there are two options: The direct reaction of a polyol with a polyisocyanate (one-step process) and the two-step process. In the two-stage process, two prepolymers are produced in the first step : with diisocyanates in excess, an NCO prepolymer is obtained when reacting with diols and an OH prepolymer is obtained when reacting with an excess of diols. Only in the second step does the actual polymerization take place by mixing the prepolymers. The two-stage process leads to a very wide-meshed crosslinking of the polymer and is important for flexible PUR foams.
Foaming
If a smaller amount of water is added to the reaction mixture, water reacts with isocyanate groups to form the corresponding unstable carbamic acid , which decomposes to form the amine with the elimination of carbon dioxide (CO 2 ) . This amine reacts with another isocyanate group to form the corresponding substituted urea . The release of CO 2 therefore does not lead to a termination of the polymerization. The resulting carbon dioxide foams the reaction mass.
The density of the foam produced can be varied by the amount of water added .
Biogenic polyols
As a rule, both the polyols and the polyisocyanates originate from the production of petrochemical raw materials, but polyols based on vegetable oils or lignin can also be used, see polyols . Castor oil can be used as a triol in coatings.
application
Foams
Foams can be made very easily from polyurethane. The special thing about PUR foams is that processing companies take semifinished product (foam in tailored form) or foams made of liquid components in place to establish ( situ , "Formed in-place foam") can. The components can also be brought into or onto industrial parts; this is where the foam is created.
Soft PUR foams are used for a wide variety of purposes, especially as upholstery material (e.g. for furniture or car seats) as mattress foam, as carpet backing material, for textile lamination, as a cleaning sponge or as a filter material. PUR flexible foams are mostly open-cell and are available in a wide range of hardness and density.
PUR rigid foams are mainly used for thermal insulation, e.g. B. in buildings, cooling devices, heat and cold storage and some pipe systems ( plastic jacket composite pipe , flexible composite pipes ) used.
There are other, relatively new areas of application for PUR foams in vehicle construction (steering wheel, armrest, soft coating of handles, interior trim, dashboard, sound insulation, rattle protection, seals, transparent coating of wood decors).
Polyurethane foams, which are designed as thermal insulation, have a closed-cell structure so that the cell gases with their low thermal conductivity remain in the foam cells. In the past, R 11 ( trichlorofluoromethane ) was often used as the cell gas. Because of the ozone-damaging properties of this halogenated hydrocarbon, it was largely replaced by carbon dioxide and currently by cyclopentane , with a mixture of cyclopentane (approx. 10 to 35%) and carbon dioxide then being contained in the foam cells. If the polyurethane foam is not encapsulated in a diffusion- proof manner with respect to the environment, the cell gases originally present are gradually replaced by air and water vapor through diffusion processes , whereby the thermal conductivity of the polyurethane foam increases. After production, polyurethane foams with carbon dioxide as the cell gas achieve thermal conductivities of approx. 0.029 to 0.033 W m −1 K −1 , while polyurethane foams with cyclopentane as the cell gas achieve thermal conductivities of approx. 0.022 to 0.027 W m −1 K - 1 . The polyurethane foams can be set both hard and flexible with different densities .
PU rigid foam panels are available in different densities. Some of the products have fillers ( glass microballoons , aluminum powder ). Intended use are insulation materials as well as model and fixture construction . The foam is usually machined for this purpose .
In the past, polyurethane foams were flame retardant with pentabromodiphenyl ether . Because of the toxicity of this substance, other flame retardants such as TCPP or expandable graphite are used today .
Paints, coatings and adhesives
One of the most important uses of polyurethanes is in paints and coatings. Here, because of their good adhesion properties , polyurethanes are used as primers and because of their high resistance to solvents, chemicals and weathering as top and clear coats in many areas of application. These include B. also coil coating lacquers and coatings for floors . Textile coatings and finishes as well as leather finishing should also be mentioned . Flat applications for bonding different, preferably flexible materials (in the area of shoes, wood / furniture, automobile interiors) are also an important area of application for polyurethane systems. In medicine, polyurethanes are used as liners in prosthetics of the lower extremities.
Liquid systems, such as moisture-curing prepolymers, 2-component systems, high solids, polyurethane solutions and polyurethane dispersions , but also solids, e.g. B. Granules (TPUs) or powders that are melted or dissolved.
Casting compounds
- PU vacuum casting resins : Various products with a short pot life , mostly for prototypes or pre-series, e.g. B. Series materials ( thermoplastic - injection molding : ABS , PP , POM , PS , PC , PMMA , etc.) correspond resembling mechanical and thermal specifications or visual aspects. They are processed in a vacuum casting machine. Molds usually made of polyaddition-curing silicone . For example, for the duplication of parts manufactured using rapid prototyping techniques .
- PU high-speed casting resins: relatively easy to process products for cast parts, models and tools that have a short pot life and do not have to be processed under vacuum.
- Elastomer curing PU casting resins: Products with different degrees of hardness in the Shore A and Shore D range. For elastic to hard elastic parts, molds and tools.
- Electrical casting compounds: for encapsulating / sheathing electrical and electronic components (potting) for the purpose of electrical insulation and protection against aggressive environmental conditions (chemical, temperature, vibrations, mechanical)
- Edge casting compounds: for casting around / wrapping wood / MDF. With polyurethane as the edge potting material, there is reliable protection against knocks, scratches, etc. Edge potting systems can be made lightfast or lightfast. Flame protection also plays an important role, especially in public transport applications. The edge potting systems are also resistant to chemical and mechanical influences.
Special uses
Polyurethane is used to make wound pads , mattresses , shoe soles , seals , hoses , floors , insulating materials , varnishes , adhesives , sealants , skis , car seats , running tracks in stadiums , dashboards , casting compounds , latex-free condoms (condoms), cast floors and much more.
- In the optical industry, polyurethane filled with certain polishing agents (e.g. ceria ) is used for the CNC polishing of optical functional surfaces.
- In the laboratory equipment industry, polyurethane is used as a material for coating volumetric flasks . The usage temperature ranges from −30 to +80 ° C. Brief exposure to higher temperatures of up to 135 ° C is permitted, but in the long run this leads to a reduction in elasticity.
- Book spines are glued with polyurethane in postpress.
- Polyurethane is used in construction as a 1- or 2-component foam ( assembly foam , expansion foam ) for sealing joints in concrete before pouring, for stabilizing foundations, for lifting parts of buildings, floors, etc. and for installing windows and doors . In the Netherlands in particular, it is also used as flooring in residential buildings.
- Rigid polyurethane foam is used as an insulating and insulating layer in sandwich elements. The elements consist of an inner and outer sheet (aluminum or coated steel sheet), with the space in between being filled by the swelling PU foam. These sandwich elements are mainly used in industrial construction in system halls, as they are prefabricated and can also be quickly assembled. In this way, wall and roof constructions are created in a short time, which are insulated and immediately finished inside and outside. Sandwich elements are also used in insulated roller and sliding doors (garage doors). In addition, rigid PUR foam is used for protection against the cold, as this foam slows down or prevents vapor diffusion. Usually, the pipes are coated with sheet metal (stainless steel, galvanized steel, aluminum, galvanized aluminum or aluminized sheet steel), similar to the sandwich method, and then filled with the two-component foam.
- PU elastomer is often used for textile fibers. These fibers are not necessarily made from 100% polyurethane. Polyurethane is also used as microfoam for breathable membranes for rainwear.
- Due to their excellent mechanical properties, certain polyurethanes are suitable for applications that require high wear resistance . So z. B. when transporting bulk goods through polyurethane hoses, or as a protective layer in pipes and pipe bends. It is also used as a sheathing for electrical cables (e.g. extension cables), for example in the popular H07BQ-F cable.
- Another more special industrial processing spectrum can be found in prototype and sample construction as well as in the foundry industry . Here products made of polyurethane are used to manufacture models and tools of all kinds, but also series parts.
- Polyurethane is used as a filler in the manufacture of multifilament tennis strings .
- Modern footballs (e.g. Roteiro) are made entirely of polyurethane.
- The outer shell of a bowling ball is made of polyurethane.
- High-quality rubber boots are now also often made of polyurethane, as they are much lighter and more elastic at low temperatures than those made of PVC. In addition, the foamed polyurethane offers far better insulation against the cold.
- Condoms / condoms without latex are made of polyurethane. These are thinner and should be more "feeling" and are well tolerated by people with latex allergies. Compared to the usual latex condoms, however, they are often more expensive (as of early 2011). PUR is stronger, but less flexible than latex.
- It is used more and more often as a coating for silicone implants because the tissue bonds well with it.
- The first production vehicle with a complete polyurethane body is the Artega GT .
- Many process steps are necessary for the manufacture of semiconductor wafers . In order to ensure an even surface, the wafers are repeatedly polished in the meantime (see CMP, chemical mechanical polishing). In most cases, the polishing plate consists of a polyurethane-coated plastic. Small polishing particles that are placed between the polishing pad and the wafer ensure abrasion.
- In the manufacturing jewelry industry, PU is used as an insert for various chains (neck, hand and ankle chains), which creates a special look.
- The running surfaces of inline skates, skateboard rollers and roller coasters are made of PU, some of them are also made of conveyor belts and conveyor belts. The PU largely determines the running properties of the rollers.
- Bushings of skateboard axles are also made of PU.
- Shoe soles and standing mats in the health sector. PU makes them soft and elastic.
- One of the two components of the Alcantara imitation leather .
- In cosmetics as a component of color cosmetics, skin care, hair care and sun protection products.
Trade names
- Block material : NECURON, obomodulan, Ureol, Raku-Tool, RenShape
- Sealing compound: Betamate, Sikaflex, Dymonic NT, Raku Pur
- Fibers : Elastane (Spandex), Lycra, Dorlastan
- Rigid foams : steinothan, BauderPIR, Baytherm, Baydur, Elastolit
- Adhesives : Baycoll, Beli-Zell, Desmocoll, Sikaflex, Gorilla Glue, Delo-Pur
- Cosmetics: Baycusan ( microplastic )
- Lacquers and coatings : Lupranol, Lupranat, Bayhydrol, Bayhydur, Sikafloor, Desmodur / Desmophen (= DD lacquers), Voranol, Voranate, Suprasec, Basonat, Sovermol, Tolonate, Duranate
- Membranes: Dermizax
- Polyester-urethane rubber : Baytec, Cellasto, Vulkollan , Elasturan, Sylomer, Sylodyn, Urepan , Regufoam
- PU films: Walopur, Walotex, Platilon
- Thermoplastic polyurethanes: Elastollan, Desmopan
- Potting compounds : Arathane (electronics), Baygal / Baymidur (electrical and electronic potting compounds ), Bectron (electronics), Elastocoat, Fermadur, RAKU PUR potting compound (electronics), Stobicast (electrical engineering, electronics), WEVO potting compound (electronics), Wepuran- Casting compound (electronics)
- Soft foams : Bayflex, Elastoflex, Elastofoam, Fermapor K31, Plasthan, RAKU PUR sealing foam
Norms
- EN 13165 Thermal insulation products for buildings - Factory made rigid polyurethane foam (PU) products - Specification .
See also
literature
- Reinhard Leppkes: Polyurethanes - a material with many faces . 5th edition. Verlag Moderne Industrie, 2003 ISBN 3-478-93100-2 .
- Karl Oberbach: Saechtling plastic pocket book . 28th edition. Hanser, 2001 ISBN 3-446-21605-7 .
- Günter Oertel (Ed.): Kunststoff-Handbuch - Vol. 7 Polyurethane. 3. Edition. Carl Hanser Verlag, 1993 ISBN 3-446-16263-1 .
- DC Allport, DS Gilbert, SM Outterside (Eds.): MDI and TDI: safety, health and environment. A source book and practical guide. John Wiley & Sons Ltd., 2003, ISBN 0-471-95812-3 .
- Karl F. Berger, Sandra Kiefer (eds.): Seal Technology Yearbook 2007. ISGATEC, Mannheim 2006, ISBN 978-3-9811509-0-2 .
- Konrad Uhlig: Polyurethane pocket book: with 34 tables , Hanser-Verlag, Munich and Vienna 3rd edition 2006, ISBN 978-3-446-40307-9 .
- Karl Hübner: 75 years of polyurethanes - “You are probably not the right man” . In: Chemistry in Our Time . tape 46 , no. 2 , 2012, p. 120–122 , doi : 10.1002 / ciuz.201290014 .
- Bodo Müller, Walter Rath: Formulation of adhesives and sealants Vincentz Network, Hannover, Germany, 2nd edition 2009, ISBN 978-3-86630-818-3
Individual evidence
- ↑ Otto Bayer: The di-isocyanate polyaddition process (polyurethanes) . In: Angewandte Chemie . 59, No. 9, 1947, pp. 257-72. doi : 10.1002 / anie.19470590901 .
- ↑ patent DE728981 : Inventor: IG Farben , 1937.
- ^ Raymond B. Seymour, George B. Kauffman: Polyurethanes: A class of modern versatile materials . In: Journal of Chemical Education . 69, No. 11, 1992, p. 909. bibcode : 1992JChEd..69..909S . doi : 10.1021 / ed069p909 .
- ↑ G. Avar: Polyurethane (PUR). In: plastics. No. 10, 2008, pp. 205-211.
- ↑ ISOPA - European Association of Diisocyanate and Polyol Manufacturers.
- ^ Wolfgang Kaiser : Synthetic chemistry for engineers. 2nd edition, Hanser, Munich 2006, ISBN 978-3-446-41325-2 .
- ↑ PUR, if possible without VOC Vincentz-Verlag, Farbe & Lack, 05/2006, page 36. Retrieved on October 29, 2017.
- ↑ M. Rampf, O. Speck, T. Speck, RH Luchsinger: Investigation of a fast mechanical self-repair mechanism for inflatable structures. In: International Journal of Engineering Science. 63, 2013, p. 61, doi : 10.1016 / j.ijengsci.2012.11.002 .
- ↑ unknown: products. In: Adhäsion KLEBEN & DICHTEN. 56, 2012, p. 46, doi : 10.1365 / s35145-012-0099-1 .