User:Sebquantic/AO notes and Rabies: Difference between pages
Sebquantic (talk | contribs) No edit summary |
m Reverted edits by 67.166.164.198 (talk) to last version by ChyranandChloe |
||
| Line 1: | Line 1: | ||
{{otheruses}} |
|||
==TODO list for [[Amon Tobin]]== |
|||
{{tooshort}} |
|||
[[Wikipedia:Citation Templates]] |
|||
{{Taxobox | color=violet |
|||
*integrate these sources |
|||
| name = ''Rabies virus'' |
|||
http://findarticles.com/p/articles/mi_m0EIN/is_2007_Sept_4/ai_n19494286?tag=content;col1 |
|||
| image = Elec_micro_of_rahbdovirus_isolate.jpg |
|||
| image_width = 200px |
|||
| image_caption = ''EM of rabies virus.'' |
|||
| virus_group = v |
|||
| ordo = ''[[Mononegavirales]]'' |
|||
| familia = ''[[Rhabdoviridae]]'' |
|||
| genus = ''[[Lyssavirus]]'' |
|||
| species = '''Rabies virus''' |
|||
}} |
|||
{{Infobox_Disease |
|||
| Name = Rabies |
|||
| DiseasesDB = 11148 |
|||
| ICD10 = {{ICD10|A|82||a|82}} |
|||
| ICD9 = {{ICD9|071}} |
|||
| ICDO = |
|||
| OMIM = |
|||
| MedlinePlus = 001334 |
|||
| eMedicineSubj = med |
|||
| eMedicineTopic = 1374 |
|||
| eMedicine_mult = {{eMedicine2|emerg|493}} {{eMedicine2|ped|1974}} |
|||
| MeshID = D011818 |
|||
}} |
|||
'''Rabies''' (from {{lang-la|rabies}}, “madness, rage, fury.” Also known as “'''hydrophobia'''”) is a [[virus (biology)|viral]] [[zoonotic]] [[neurotropic virus|neuroinvasive]] disease that causes acute [[encephalitis]] (inflammation of the [[brain]]) in mammals. It is most commonly caused by a bite from an infected animal, but occasionally by other forms of contact. |
|||
http://www.soundonsound.com/sos/Apr03/articles/amontobin.asp |
|||
<!-- Need more detail here on symptons, carriers etc. to make the lead a reasonable overview of the article. --> |
|||
The rabies virus makes its way to the brain from the site of infection by following the [[Peripheral nervous system|peripheral nerves]].<ref name="Robbins">{{cite book |author=Mitchell RS, Kumar V, Robbins SL, Abbas AK, Fausto N|title=Robbins basic pathology |publisher=Saunders/Elsevier |location= |year=2007 |pages= |isbn=1-4160-2973-7}}</ref> The [[incubation period]] of the disease depends on how far the virus must travel to reach the [[central nervous system]], usually taking a few months.<ref name="Robbins" /> |
|||
<!-- <ref>{{cite web | url= | title= | accessdate=2008-10-03 | publisher= | date= | author= | pages= | format= }}</ref> --> |
|||
In the beginning stages of rabies, the symptoms are [[malaise]], headache, and [[fever]], while in later stages it includes acute pain, violent movements, and the inability to swallow water (hence the name ''hydrophobia'')<ref name="Robbins" />. In the final stages, the patient begins to have periods of [[mania]] and [[lethargy]], and coma.<ref name="Robbins" /> Death generally occurs due to respiratory insufficiency.<ref name="Robbins" /> |
|||
*propose that somebody do a complete "Amon Tobin Discography" page modeled after [[WikiProject Discography]] |
|||
In non-vaccinated humans, rabies is almost invariably fatal after [[neurological]] symptoms have developed, but prompt post-exposure [[vaccination]] may prevent the virus from progressing. There are only six known cases of a person surviving symptomatic rabies, and only one known [[#Induced coma treatment|case]] of survival in which the patient received no rabies-specific treatment either before or after illness onset.<ref>{{cite journal |author= |title=Recovery of a patient from clinical rabies--Wisconsin, 2004 |journal=MMWR. Morbidity and mortality weekly report |volume=53 |issue=50 |pages=1171–3 |year=2004 |month=December |pmid=15614231 |doi= |url=http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5350a1.htm}}</ref> |
|||
===get reliable sources for these things=== |
|||
*Scored soundtrack for transporter 3 |
|||
== Virology == |
|||
*He has performed in front of more than sixty thousand people. |
|||
{{expand|date=October 2008}} |
|||
=== Classification === |
|||
The rabies virus is a member of the ''[[Lyssavirus]]'' [[genus]]. This genus of [[RNA virus]]es also includes the Aravan virus, [[Australian bat lyssavirus]], [[Duvenhage virus]], European bat lyssavirus 1, European bat lyssavirus 2, Irkut virus, Khujand virus, [[Lagos bat virus]], [[Mokola virus]] and the West Caucasian bat virus. |
|||
*released song as 60hz |
|||
=== Structure === |
|||
*Usually, at least one of his tracks is used in the British motor show [[Top Gear (current format)|Top Gear]] |
|||
Lyssaviruses have [[helical]] symmetry, so their infectious particles are approximately cylindrical in shape. This is typical of plant-infecting viruses; human-infecting viruses more commonly have cubic symmetry and take shapes approximating [[regular polyhedron|regular polyhedra]]. ''[[Negri bodies]]'' in the infected neurons are [[pathognomonic]]. |
|||
==TODO list for [[Anarchy Online]]== |
|||
*move to [[cite press release]] and [[cite book]] where needed |
|||
The virus has a bullet-like shape with a length of about [[1 E-7 m|180 nm]] and a cross-sectional diameter of about [[1 E-8 m|75 nm]]. One end is rounded or conical and the other end is planar or concave. The [[lipoprotein]] envelope carries knob-like spikes composed of [[Glycoprotein]] G. Spikes do not cover the planar end of the virion (virus particle). Beneath the envelope is the membrane or matrix (M) protein layer which may be [[invaginated]] at the planar end. The core of the virion consists of helically arranged [[ribonucleoprotein]]. |
|||
=== Genome === |
|||
The [[genome]] is unsegmented linear [[antisense]] [[RNA]]. Also present in the [[nucleocapsid]] are RNA dependent RNA transcriptase and some structural proteins. |
|||
=== Life cycle === |
|||
From the source of wound of entry, the rabies virus travels quickly along the neural pathways to the central nervous system. There the virus further spreads to other organs. The saliva glands located in the tissues of the mouth and cheeks, receive high concentrations and thus allowing for it to further transmitted. The rabies virus typically causes fatality within two days to five years.<ref>{{cite web |
|||
| url = http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=mmed.figgrp.3241 |
|||
| title = Life cycle of rabies |
|||
| accessdate = 2008-10-10 |
|||
| author = |
|||
| last = Baron |
|||
| first = Samuel |
|||
| authorlink = |
|||
| coauthors = |
|||
| date = |
|||
| year = |
|||
| month = |
|||
| format = |
|||
| work = |
|||
| publisher = The University of Texas Medical Branch at Galveston |
|||
| location = |
|||
| pages = |
|||
| language = |
|||
| doi = |
|||
| archiveurl = |
|||
| archivedate = |
|||
| quote = |
|||
}} |
|||
</ref><ref>{{cite web |
|||
| url = http://www.unbc.ca/nlui/wildlife_diseases_bc/rabies.htm |
|||
| title = Rabies |
|||
| accessdate = 2008-10-10 |
|||
| author = |
|||
| last = |
|||
| first = |
|||
| authorlink = |
|||
| coauthors = |
|||
| date = |
|||
| year = |
|||
| month = |
|||
| format = |
|||
| work = |
|||
| publisher = University of Northern British Columbia |
|||
| location = |
|||
| pages = |
|||
| language = |
|||
| doi = |
|||
| archiveurl = |
|||
| archivedate = |
|||
| quote = |
|||
}} |
|||
</ref> |
|||
== Prevention == |
|||
Rabies can be prevented by vaccination, both in humans and other animals. Virtually every infection with rabies resulted in death, until [[Louis Pasteur]] and [[Emile Roux]] developed the first rabies vaccination in 1885. This vaccine was first used on a human on [[July 6]], [[1885]] – nine-year old boy [[Joseph Meister]] (1876–1940) had been mauled by a rabid dog.<ref>{{cite journal |author=Geison GL |title=Pastuer's work on rabies: Reexamining the ethical issues diagnosis for developing countries |journal=Hastings Center Report |issue=April |pages=26- |year=1978 |url=http://www.jstor.org/pss/3560403}}</ref> |
|||
Their vaccine consisted of a sample of the virus harvested from infected (and necessarily dead) rabbits, which was weakened by allowing it to dry for 5 to 10 days. Similar nerve tissue-derived vaccines are still used now in some countries, and while they are much cheaper than modern cell culture vaccines, they are not as effective and carry a certain risk of neurological complications. |
|||
The human [[diploid]] cell rabies vaccine (H.D.C.V.) was started in 1967. Human diploid cell rabies vaccines are made using the attenuated Pitman-Moore L503 strain of the virus. Human diploid cell rabies vaccines have been given to more than 1.5 million humans [[as of 2006]]. Newer and less expensive purified chicken embryo cell vaccine, and purified [[Vero cell]] rabies vaccine are now available. The purified Vero cell rabies vaccine uses the attenuated Wistar strain of the rabies virus, and uses the Vero cell line as its host. |
|||
Some recent works have shown that during lethal rabies infection the [[blood-brain barrier]] (BBB) does not allow anti-viral immune cells to enter the brain, the primary site of rabies virus replication.<ref name="Roy_2007a">{{cite journal |author=Roy A, Phares TW, Koprowski H, Hooper DC |title=Failure to open the blood-brain barrier and deliver immune effectors to central nervous system tissues leads to the lethal outcome of silver-haired bat rabies virus infection |journal=J. Virol. |volume=81 |issue=3 |pages=1110–8 |year=2007 |pmid=17108029 |doi=10.1128/JVI.01964-06}}</ref> This aspect contributes to the pathogenicity of the virus and artificially increasing BBB permeability promotes viral clearance.<ref name="Roy_2007b">{{cite journal |author=Roy A, Hooper DC |title=Lethal silver-haired bat rabies virus infection can be prevented by opening the blood-brain barrier|journal=J. Virol. |volume=81 |issue=15 |pages=7993–8 |year=2007 |pmid=17507463 | doi = 10.1128/JVI.00710-07}}</ref> Opening the BBB during rabies infection has been suggested as a possible novel approach to treat the disease. |
|||
=== Post-exposure prophylaxis === |
|||
Treatment after exposure, known as [[post-exposure prophylaxis]] or “P.E.P.”, is highly successful preventing the disease if administered promptly, within six days after infection and consists of over a 28 day period. Thoroughly washing the wound as soon as possible with soap and water for approximately five minutes is very effective at reducing the number of viral particles. “If available, a virucidal antiseptic such as povidone-iodine, iodine tincture, aqueous iodine solution or alcohol (ethanol) should be applied after washing.”<ref>Rabies & Australian bat lyssavirus information sheet http://www.health.vic.gov.au/ideas/bluebook/rabies_info</ref> Exposed mucous membranes such as eyes, nose or mouth should be flushed well with water. In the United States, patients receive one dose of [[immunoglobulin]] and five doses of rabies vaccine over a twenty-eight day period. One-half the dose of immunoglobulin is injected in the region of the bite, if possible, with the remainder injected [[intramuscular injection|intramuscularly]] away from the bite. This is much less painful compared with administering immunoglobulin through the [[abdominal wall]] with a large needle, as was done in the past. The first dose of rabies vaccine is given as soon as possible after exposure, with additional doses on days three, seven, fourteen, and twenty-eight after the first. Patients that have previously received pre-exposure vaccination do not receive the immunoglobulin, only the post-exposure vaccinations on day 0 and 3. Since the widespread vaccination of domestic dogs and cats and the development of effective human vaccines and immunoglobulin treatments, the number of recorded deaths in the U.S. from rabies has dropped from one hundred or more annually in the early twentieth century, to 1–2 per year, mostly caused by bat bites, which may go unnoticed by the victim and hence untreated. |
|||
P.E.P. is effective in treating rabies because the virus must travel from the site of infection through the peripheral nervous system (nerves in the body) before infecting the central nervous system (brain and spinal cord) and glands to cause lethal damage. This travel along the nerves is usually slow enough that vaccine and immunoglobulin can be administered to protect the brain and glands from infection. The amount of time this travel requires is dependent on how far the infected area is from the brain: if the victim is bitten in the face, for example, the time between initial infection and infection of the brain is very short and P.E.P. may not be successful. |
|||
=== Pre-exposure prophylaxis === |
|||
Currently pre-exposure [[immunization]] has been used on domesticated and normal non-human populations. In many jurisdictions, domestic dogs, cats, and ferrets are required to be vaccinated. A pre-exposure vaccination is also available for humans, most commonly given to veterinarians and those traveling to regions where the disease is common, such as India. Most tourists do not need such a vaccination, just those doing substantial non-urban activities. However, should a vaccinated human be bitten by a carrier, failure to receive subsequent post-exposure treatment could be fatal, although post-exposure treatment for a vaccinated human is far less extensive than that which would normally be required by one with no pre-exposure vaccination. |
|||
In 1984 researchers at the [[Wistar Institute]] developed a [[recombinant]] vaccine called V-RG by inserting the [[glycoprotein]] gene from rabies into a [[vaccinia]] virus.<ref name="Wiktor_1984">{{cite journal |author=Wiktor TJ, Macfarlan RI, Reagan KJ, Dietzschold B, Curtis PJ, Wunner WH, Kieny MP, Lathe R, Lecocq JP, Mackett M |title=Protection from rabies by a vaccinia virus recombinant containing the rabies virus glycoprotein gene |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=81 |issue=22 |pages=7194–8 |year=1984 |pmid=6095272 | doi = 10.1073/pnas.81.22.7194}}</ref> The V-RG vaccine has since been commercialised by [[Merial]] under the trademark [http://www.raboral.com/ Raboral]. It is harmless to humans and has been shown to be safe for various species of animals that might accidentally encounter it in the wild, including birds (gulls, hawks, and owls).<ref name="Artois_1990">{{cite journal |author=Artois M, Charlton KM, Tolson ND, Casey GA, Knowles MK, Campbell JB |title=Vaccinia recombinant virus expressing the rabies virus glycoprotein: safety and efficacy trials in Canadian wildlife |journal=Can. J. Vet. Res. |volume=54 |issue=4 |pages=504–7 |year=1990 |pmid=2249183}}</ref> |
|||
V-RG has been successfully used in the field in [[Belgium]], [[France]], and the [[United States]] to prevent outbreaks of rabies in [[wildlife]]. The virus is stable under relatively high temperatures and can be delivered orally, making mass vaccination of wildlife possible by putting it in baits. The plan for immunization of normal populations involves dropping bait containing food wrapped around a small dose of the live virus. The bait would be dropped by helicopter concentrating on areas that have not been infected yet. Just such a strategy of oral immunization of foxes in Europe has already achieved substantial reductions in the incidence of human rabies. A strategy of vaccinating “neighborhood dogs” in [[Jaipur]], [[India]], (combined with a sterilization program) has also resulted in a large reduction in the number of human cases.<ref name="Reece_2006">{{cite journal | author=Reece JF, Chawla SK. | title=Control of rabies in Jaipur, India, by the sterilisation and vaccination of neighbourhood dogs. | journal=Vet Rec | year=2006 | volume=159 | pages=379–83 }}</ref> |
|||
== Symptoms == |
|||
The period between infection and the first [[flu]]-like symptoms is normally two to twelve weeks, but can be as long as two years. Soon after, the symptoms expand to slight or partial [[paralysis]], [[cerebral dysfunction]], [[anxiety]], [[insomnia]], [[confusion]], [[agitation (emotion)|agitation]], abnormal behavior, [[paranoia]], terror, [[hallucination]]s, progressing to [[delirium]].{{Fact|date=February 2008}} The production of large quantities of saliva and tears coupled with an inability to speak or swallow are typical during the later stages of the disease; this can result in “[[hydrophobia]]”, where the victim has difficulty swallowing because the throat and jaw become slowly paralyzed, shows panic when presented with liquids to drink, and cannot quench his or her thirst. The disease itself was also once commonly known as ''[[wiktionary:hydrophobia|hydrophobia]]'', from this characteristic symptom. The patient “foams at the mouth” because they cannot swallow their own saliva for days and it gathers in the mouth until it overflows. |
|||
Death almost invariably results two to ten days after the first symptoms; the few humans who are known to have survived the disease {{Fact|date=July 2008}} were all left with severe [[brain damage]], with the recent exception of [[Jeanna Giese]] (see below). It is [[neurotropic virus|neurotropic]] in nature. |
|||
In ''Rabies: The Facts''<ref>{{cite book |author=Warrell, D. A.; Kaplan, Colin; Turner, G. S. |title=Rabies: the facts |publisher=Oxford University Press |location=Oxford [Oxfordshire] |year=1986 |pages=pp. 43-5 |isbn=0-19-261441-X |oclc= |doi= |accessdate=}}</ref>, Kaplan et. al. describe several typical cases, including one of a 23 year-old Englishwoman: |
|||
<blockquote>“On June 17, 1981 she was bitten on the ankle by a dog in New Delhi. On August 18, about two months later, she experienced the first prodromal symptoms. She became anxious and depressed, and it became impossible for her to drink more than small sips of liquid. While sleeping, she frequently sat up in bed suddenly, terrified. On August 19, she became confused, hallucinated, and was incontinent of urine. On August 20, she was unable to eat or drink and was taken to the hospital where she hallucinated and screamed in terror. Misdiagnosed as a psychiatric case, she was injected with a tranquilizer and sent home, however she repeatedly woke up screaming in fear and became so wild and agitated that her husband felt he could not deal with her by himself and took her to her mother's house. She remained terrified, hallucinating and screaming in horror throughout the night. She had no water for almost three days. She fell into a coma the next morning, and died on August 23.”</blockquote> |
|||
== Diagnosis == |
|||
The [[differential diagnosis]] in a case of suspected human rabies may initially include any cause of encephalitis, |
|||
particularly infection with viruses such as [[herpesviridae|herpesviruses]], [[enteroviruses]], and [[arboviruses]] (e.g., [[West Nile virus]]). The most important viruses to rule out are [[herpes simplex virus]] type 1, [[varicella-zoster virus]], and (less commonly) enteroviruses, including [[coxsackie virus|coxsackievirus]]es, [[echovirus]]es, [[poliovirus]]es, and human [[enterovirus]]es 68 to 71. A specific diagnosis may be made by a variety of diagnostic techniques, including [[polymerase chain reaction]] (PCR) testing of [[cerebrospinal fluid]], [[cell culture#Viral culture methods|viral culture]], and [[serology]]. In addition, consideration should be given to the local [[epidemiology]] of encephalitis caused by arboviruses belonging to several [[taxonomy|taxonomic]] groups, including eastern and western [[equine encephalitis|equine encephalitis virus]]es, [[St. Louis encephalitis]] virus, [[Powassan virus]], the [[California encephalitis virus]] serogroup, and [[La Crosse virus]]. |
|||
New causes of viral encephalitis are also possible, as was evidenced by the recent outbreak in Malaysia of some 300 cases of encephalitis (mortality rate, 40%) caused by [[Nipah virus]], a newly recognized [[paramyxovirus]].<ref name="refDiseasesOfSwine">{{cite book |author=Taylor DH, Straw BE, Zimmerman JL, D'Allaire S |title=Diseases of swine |publisher=Blackwell publishing |location=Oxford |year=2006 |pages= |isbn=0-8138-1703-X |url=http://books.google.com/books?id=3o9l77HdZkgC&dq=diseases+of+swine |doi= |accessdate=2008-10-05}}</ref> Similarly, well-known viruses may be introduced into new locations, as is illustrated by the recent outbreak of encephalitis due to West Nile virus in the eastern United States.<ref>{{cite book |title=Inflammatory Disorders Of The Nervous System: Pathogenesis, Immunology, and Clinical Management |last=Minagar |first=Alireza |coauthors=J. Steven Alexander |year=2005 |publisher=Humana Press |isbn=1588294242 }}</ref> Epidemiologic factors (e.g., season, geographic location, and the patient’s age, travel history, and possible exposure to animal bites, rodents, and ticks) may help direct the diagnostic workup. |
|||
Cheaper rabies diagnosis will be possible for low-income settings according to research reported on the Science and Development Network website in 2008. Accurate rabies diagnosis can be done ten times cheaper, according to researchers from the Farcha Veterinary and Livestock Research Laboratory and the Support International Health Centre in N'Djamena, Chad. The scientists evaluated a method using light microscopy, cheaper than the standard tests, and say this could provide better rabies control across Africa.<ref>{{cite journal |author=Dürr S, Naïssengar S, Mindekem R, ''et al'' |title=Rabies diagnosis for developing countries |journal=PLoS neglected tropical diseases |volume=2 |issue=3 |pages=e206 |year=2008 |pmid=18365035 |pmc=2268742 |doi=10.1371/journal.pntd.0000206 |url=http://www.plosntds.org/article/info:doi/10.1371/journal.pntd.0000206}}</ref> |
|||
== Treatments == |
|||
=== Induced coma === |
|||
{{main|Jeanna Giese}} |
|||
In 2005, the case of Jeanna Giese, a girl of 15 who survived acute, invaccinated rabies was reported, indicating the successful treatment of rabies through [[induced coma|induction of a coma]].<ref name="Willoughby_2005">{{cite journal |author=Willoughby RE, Tieves KS, Hoffman GM, Ghanayem NS, Amlie-Lefond CM, Schwabe MJ, Chusid MJ, Rupprecht CE |title=Survival after treatment of rabies with induction of coma |journal=N. Engl. J. Med. |volume=352 |issue=24 |pages=2508–14 |year=2005 |pmid=15958806 | doi = 10.1056/NEJMoa050382}}</ref> This treatment approach was based on the theory that rabies' detrimental effects were caused by temporary dysfunctions of the brain, and that the induction of a coma, by producing a temporary partial stop in brain function, would protect the brain from damage while the body built up an immune response to the virus. After thirty-one days of isolation and seventy-six days of hospitalisation, she was released from the hospital, having survived rabies. |
|||
Rodney Willoughby Jr., the primary care physician in this case published in the April 2007 issue of ''Scientific American''.<ref name="SciAmApr07">Rodney E. Willoughby, Jr., “A Cure for Rabies?” ''Scientific American'', V. 256, No. 4, April 2007, p. 95 ([http://sciam.com/article.cfm?chanID=sa006&colID=1&articleID=5BCA4D82-E7F2-99DF-33323DF6FF21871F online link])</ref> He notes that subsequent failures of what he calls the ''Milwaukee protocol'' did not use the cocktail of drugs used during the treatment of Giese. A point he makes for future research is the relationship of the virus to depletion of [[biopterin]] in the brain. |
|||
Later attempts to use the same treatment have failed, but in April, 2008, in [[Cali]], [[Colombia]], it was reported (by local newspapers) that an 11-year-old may have recovered successfully after induction of coma <ref name="El">El Tiempo Nación Cali, “Nuevos síntomas dan aliento sobre recuperación de niño caucano contagiado por rabia,” April 10th 2008([http://www.eltiempo.com/nacion/cali/2008-04-08/ARTICULO-WEB-NOTA_INTERIOR-4081557.html])</ref>. This patient was infected on February 15 when several children were bitten by a cat in Santander de Quilichao, a small town near Cali. However, this claim has not been verified. |
|||
== Epidemiology == |
|||
=== Vectors === |
|||
[[Image:Rabies Virus EM PHIL 1876.JPG|thumb|[[Transmission electron microscopy|TEM]] [[micrograph]] with numerous rabies [[virion]]s (small dark-grey rod-like particles) and Negri bodies (the larger [[pathognomonic]] cellular inclusions of rabies infection).]] Any mammal may become infected with the rabies virus and develop symptoms, including humans. Most animals can be infected by the virus and can transmit the disease to humans. Infected [[bat]]s, [[monkey]]s, [[raccoon]]s, [[fox]]es, [[skunk]]s, [[cattle]], [[wolf|wolves]], [[dog]]s or [[cat]]s provide the greatest risk to humans. Rabies may also spread through exposure to infected [[livestock|domestic farm animals]], [[groundhog]]s, [[weasel]]s and other [[Carnivora|wild carnivores]]. [[Rodent]]s ([[mouse|mice]], [[squirrel]]s etc) are seldom infected. |
|||
The virus is usually present in the nerves and [[saliva]] of a symptomatic rabid animal.<ref>The ''Merck Manual'', Eleventh Edition (1983), p. 183</ref><ref>''The Merck manual of Medical Information. Second Home Edition'', (2003), p. 484.</ref> The route of [[infection]] is usually, but not necessarily, by a bite. In many cases the infected animal is exceptionally aggressive, may attack without provocation, and exhibits otherwise uncharacteristic behaviour[http://www.nda.agric.za/docs/rabies/rabies.htm]. Transmission may also occur via an [[particulate|aerosol]] through [[mucous membrane]]s; transmission in this form may have happened in people exploring caves populated by rabid bats. |
|||
Transmission between humans is extremely rare, although it can happen through [[organ transplant|transplant surgery]] (see below for recent cases), or, even more rarely, through bites, kisses or sexual relations. |
|||
After a typical human infection by bite, the virus enters the [[peripheral nervous system]]. It then travels along the [[nerve]]s towards the [[central nervous system]]. During this phase, the virus cannot be easily detected within the host, and vaccination may still confer cell-mediated immunity to prevent symptomatic rabies. Once the virus reaches the [[brain]], it rapidly causes [[encephalitis]]. This is called the “prodromal” phase. At this time, treatment is useless. Then symptoms appear. Rabies may also inflame the [[spinal cord]] producing [[myelitis]]. |
|||
=== Prevalence === |
|||
{{expand-section|information from the main article|date=October 2008}} |
|||
{{main|Prevalence of rabies}} |
|||
[[Image:Rabies Free Countries.svg|thumb|right|300px|Rabies-free jurisdictions, as of January 2006: |
|||
[[Australia]], [[New Zealand]], [[Singapore]], [[Fiji]], [[Guam]], [[Hawaii]], the [[United Kingdom]], [[Republic of Ireland]], [[Norway]], [[Sweden]], [[Finland]], [[Iceland]], [[Japan]] and [[Taiwan]]/ROC.]] |
|||
Almost all human deaths caused by rabies originate from Asia and Africa. There are an estimated 55,000 human deaths annually from rabies worldwide, with about 31,000 in Asia, and 24,000 in Africa.<ref>“Rabies” (2006) World Health Organisation. [http://www.who.int/mediacentre/factsheets/fs099/en/]</ref> |
|||
One of the sources of recent flourishing of rabies in [[East Asia]] is the pet boom. [[China]] introduced in the city of [[Beijing]] the “[[one-dog policy]]” in November 2006 to control the problem.<ref>[http://www.thestar.com/News/article/238729 ''The Toronto Star'' “China cracks down on rabid dog menace”]</ref> India has been reported as having the highest rate of human rabies in the world [http://www.independent.co.uk/news/science/dead-as-a-dodo-why-scientists-fear-for-the-future-of-of-the-asian-vulture-818059.html]. |
|||
=== Rabies and animals === |
|||
{{expand-section|information from the main article|date=October 2008}} |
|||
{{main|Rabies and animals}} |
|||
Rabies is infectious to mammals. |
|||
In dogs three stages of rabies are recognized in dogs and other animals. The first stage is a one to three day period characterized by behavioral changes and is known as the [[Prodrome|prodromal stage]]. The second stage is the excitative stage, which lasts three to four days. It is this stage that is often known as ''furious rabies'' due to the tendency of the affected dog to be hyperreactive to external stimuli and bite at anything near. The third stage is the paralytic stage and is caused by damage to [[motor neuron]]s. Incoordination is seen due to rear limb [[paralysis]] and drooling and difficulty swallowing is caused by paralysis of facial and throat muscles. Death is usually caused by [[respiratory arrest]].<ref name=Ettinger_1995>{{cite book|author=Ettinger, Stephen J.;Feldman, Edward C.|title=Textbook of Veterinary Internal Medicine|edition=4th ed.|publisher=W.B. Saunders Company|year=1995|id=ISBN 0-7216-6795-3}}</ref> |
|||
=== Recent cases === |
|||
Several recently publicized cases have stemmed from bats, which are known to be a [[Vector (biology)|vector]] for rabies. See the [[#Rabies vectors]] section below. |
|||
In October 2004 a female brown [[bear]] killed one human and injured several others near the city of [[Braşov]] in Central [[Romania]]. The bear was killed by human hunters and diagnosed with rabies. More than one hundred humans were vaccinated afterwards. |
|||
[[Image:Rabies patient.jpg|thumb|right|250px|A patient with rabies, 1959.]] |
|||
Rabies is known to have been transmitted between humans by [[Organ transplant|transplant surgery]] on rare occasions. |
|||
Infections by [[cornea]]l transplant have been reported in Thailand (two cases), India (two cases), Iran (two cases),<ref name="Javadi_1996">{{cite journal | author=Javadi MA, Fayaz A, Mirdehghan SA, Ainollahi B | title=Transmission of rabies by corneal graft | journal=Cornea | year=1996 | pages=431–3 | volume=15 | issue=4 | pmid=8776570 | doi = 10.1097/00003226-199607000-00014}}</ref> the United States (one case), and France (also a single case).<ref>{{cite journal | author = CDC | title = Human-to-human transmission of rabies via a corneal transplant -- France | journal = MMWR | year = 1980 | volume = 29 | issue = | pages = 25–6 | url= }}</ref> Details of two further cases of infection resulting from corneal transplants were described in 1996. |
|||
In June 2004, three organ recipients died in the United States from rabies transmitted in the transplanted kidneys and liver of an infected donor from [[Texarkana]].<ref name="MMWR_2004a">{{cite journal | author= | title=Investigation of rabies infections in organ donor and transplant recipients--Alabama, Arkansas, Oklahoma, and Texas, 2004 | journal=MMWR Morb Mortal Wkly Rep | year=2004 | pages=586–9 | issue=26 | pmid=15241303 | volume=53 }}</ref> There were bats near the donor's home, and the donor had told others that he had been bitten.<ref name="MMWR_2004b"> {{cite journal | author= | title=Update: investigation of rabies infections in organ donor and transplant recipients--Alabama, Arkansas, Oklahoma, and Texas, 2004 | journal=MMWR Morb Mortal Wkly Rep | year=2004 | pages=615–6 | volume=53 | issue=27 | pmid=15254455 }}</ref> The donor is now reported to have died of a cerebral hemorrhage, the culmination of an unidentified neurological disorder, although recipients are said to have been told the cause of death had been a car crash. Marijuana and cocaine were found in the donor's urine at the time of his death, according to a report in ''The New England Journal of Medicine''.<ref name="Srinivasan_2005">{{cite journal | author=Srinivasan A, Burt EC, Kuehnert MJ, Rupprecht C, Sutker WL, Ksiazek TG, Paddock CD, Guarner J, Shieh WJ, Goldsmith C, Hanlon CA, Zoretic J, Fischbach B, Niezgoda M, El-Feky WH, Orciari L, Sanchez EQ, Likos A, Klintmalm GB, Cardo D, LeDuc J, Chamberland ME, Jernigan DB, Zaki SR | title=Transmission of rabies virus from an organ donor to four transplant recipients | journal=N Engl J Med | year=2005 | pages=1103–11 | volume=352 | issue=11 | pmid=15784663 | doi = 10.1056/NEJMoa043018}}</ref><blockquote>"[The surgeons] thought he had suffered a fatal crack-cocaine overdose, which can produce symptoms similar to those of rabies. ‘We had an explanation for his condition,’ says Dr. Goran Klintmalm, a surgeon who oversees transplantation at Baylor University Medical Center, where the transplants occurred. ‘He’d recently smoked crack cocaine. He’d hemorrhaged around the brain. He’d died. That was all we needed to know.’ Because of doctor-patient confidentiality rules, doctors involved with this case would not talk about it on the record, but a few did say that if no cocaine was found in the donor’s blood, the E.R. doctors might have investigated his symptoms more aggressively instead of assuming he had overdosed. (Because no autopsy was done, doctors have not been able to establish whether the rabies or the drugs actually killed him.)"<ref> {{cite journal | author = Reynolds G | title = Will Any Organ Do? | journal = The New York Times Magazine | year = 2005 | volume = | issue = 10 July | pages = –}}</ref></blockquote> |
|||
In February 2005, three [[Germany|German]] patients in [[Mainz]] and [[Heidelberg]] were diagnosed with rabies after receiving various organs and cornea transplants from a female donor. Two of the infected people died. Three other patients who received organs from the woman have not yet shown rabies symptoms. The 26 year old donor had died of heart failure in December 2004 after consuming [[cocaine]] and [[ecstasy (drug)|ecstasy]]. In October 2004, she had visited India, one of the countries worst affected by rabies worldwide. Dozens of medical staff were vaccinated against rabies in the two hospitals as a precautionary measure. Associated Press reports that "Donated organs are never tested for rabies. The strain detected in the victims’ bodies is one commonly found in bats, health officials said." According to CNN "Rabies tests are not routine donor screening tests, Virginia McBride, public health organ donation specialist with the Health Resources and Services Administration, said. The number of tests is limited because doctors have only about six hours from the time a patient is declared brain-dead until the transplantation must begin for the organs to maintain viability." |
|||
== See also == |
|||
{{wiktionary}} |
|||
{{wikinews}} |
|||
* [[World Rabies Day]] |
|||
* [[Alliance for Rabies Control]] |
|||
* [[Neurotropic virus]] |
|||
<br clear="all"/> |
|||
== References == |
|||
{{Reflist|colwidth=30em}} |
|||
== Further reading == |
|||
{{refbegin}} |
|||
* {{cite journal |author=Waterman JA |title=The history of the outbreak of paralytic rabies in Trinidad transmitted by bats to human beings and the lower animals from 1925 |journal=Caribb Med J |volume=21 |issue= |pages=1–6 |year=1959 |pmid=13843069 |doi= |url=}} |
|||
* {{cite book |author=Fleming, Theodore H. |title=A bat man in the tropics: chasing El Duende |publisher=University of California Press |location=Berkeley |year=2003 |pages= |isbn=0-520-23606-8 |oclc= |doi= |accessdate=}} |
|||
* {{cite book |author=Warrell, D. A.; Kaplan, Colin; Turner, G. S. |title=Rabies: the facts |publisher=Oxford University Press |location=Oxford [Oxfordshire] |year=1986 |pages= |isbn=0-19-261441-X |oclc= |doi= |accessdate=}} |
|||
{{refend}} |
|||
== External links == |
|||
{{DMOZ|Health/Conditions_and_Diseases/Infectious_Diseases/Viral/Rabies/}} |
|||
{{Viral diseases}} |
|||
{{Zoonotic viral diseases}} |
|||
{{Domestic cat}} |
|||
[[Category:Cat diseases]] |
|||
[[Category:Dog diseases]] |
|||
[[Category:Rat carried diseases]] |
|||
[[Category:Mononegavirales]] |
|||
[[Category:Neurology]] |
|||
[[Category:Rabies|Rabies]] |
|||
[[Category:Viral diseases]] |
|||
[[Category:Zoonoses]] |
|||
{{Link FA|ka}} |
|||
[[ar:داء الكلب]] |
|||
[[zh-min-nan:Siáu-káu-pēⁿ]] |
|||
[[bs:Bjesnilo]] |
|||
[[bg:Бяс]] |
|||
[[cv:Тилĕрӳ]] |
|||
[[cs:Vzteklina]] |
|||
[[da:Hundegalskab]] |
|||
[[de:Tollwut]] |
|||
[[et:Marutaud]] |
|||
[[el:Λύσσα]] |
|||
[[es:Rabia]] |
|||
[[eo:Rabio]] |
|||
[[eu:Amorru]] |
|||
[[fr:Rage]] |
|||
[[hr:Bjesnoća]] |
|||
[[id:Rabies]] |
|||
[[ia:Rabies]] |
|||
[[it:Rabbia]] |
|||
[[he:כלבת]] |
|||
[[ka:ცოფი]] |
|||
[[ku:Harbûn]] |
|||
[[la:Rabies]] |
|||
[[lt:Pasiutligė]] |
|||
[[hu:Veszettség]] |
|||
[[nl:Hondsdolheid]] |
|||
[[ja:狂犬病]] |
|||
[[no:Rabies]] |
|||
[[pl:Wścieklizna]] |
|||
[[pt:Raiva (doença)]] |
|||
[[ro:Rabie]] |
|||
[[ru:Бешенство]] |
|||
[[scn:Raggia]] |
|||
[[si:ජලභීතිකාව]] |
|||
[[simple:Rabies]] |
|||
[[sk:Besnota]] |
|||
[[sl:Steklina]] |
|||
[[sr:Besnilo]] |
|||
[[fi:Vesikauhu]] |
|||
[[sv:Rabies]] |
|||
[[th:โรคพิษสุนัขบ้า]] |
|||
[[vi:Bệnh dại]] |
|||
[[tr:Kuduz]] |
|||
[[uk:Сказ]] |
|||
[[ur:مرض کلب]] |
|||
[[wa:Må d' araedje]] |
|||
[[zh:狂犬病]] |
|||
Revision as of 13:12, 10 October 2008
This article's lead section may be too short to adequately summarize the key points. |
| Rabies virus | |
|---|---|
| File:Elec micro of rahbdovirus isolate.jpg | |
| EM of rabies virus. | |
| Virus classification | |
| Group: | Group V ((−)ssRNA)
|
| Order: | |
| Family: | |
| Genus: | |
| Species: | Rabies virus
|
| Rabies | |
|---|---|
| Specialty | Infectious diseases, veterinary medicine |
Rabies (from Latin: rabies, “madness, rage, fury.” Also known as “hydrophobia”) is a viral zoonotic neuroinvasive disease that causes acute encephalitis (inflammation of the brain) in mammals. It is most commonly caused by a bite from an infected animal, but occasionally by other forms of contact.
The rabies virus makes its way to the brain from the site of infection by following the peripheral nerves.[1] The incubation period of the disease depends on how far the virus must travel to reach the central nervous system, usually taking a few months.[1]
In the beginning stages of rabies, the symptoms are malaise, headache, and fever, while in later stages it includes acute pain, violent movements, and the inability to swallow water (hence the name hydrophobia)[1]. In the final stages, the patient begins to have periods of mania and lethargy, and coma.[1] Death generally occurs due to respiratory insufficiency.[1]
In non-vaccinated humans, rabies is almost invariably fatal after neurological symptoms have developed, but prompt post-exposure vaccination may prevent the virus from progressing. There are only six known cases of a person surviving symptomatic rabies, and only one known case of survival in which the patient received no rabies-specific treatment either before or after illness onset.[2]
Virology
Classification
The rabies virus is a member of the Lyssavirus genus. This genus of RNA viruses also includes the Aravan virus, Australian bat lyssavirus, Duvenhage virus, European bat lyssavirus 1, European bat lyssavirus 2, Irkut virus, Khujand virus, Lagos bat virus, Mokola virus and the West Caucasian bat virus.
Structure
Lyssaviruses have helical symmetry, so their infectious particles are approximately cylindrical in shape. This is typical of plant-infecting viruses; human-infecting viruses more commonly have cubic symmetry and take shapes approximating regular polyhedra. Negri bodies in the infected neurons are pathognomonic.
The virus has a bullet-like shape with a length of about 180 nm and a cross-sectional diameter of about 75 nm. One end is rounded or conical and the other end is planar or concave. The lipoprotein envelope carries knob-like spikes composed of Glycoprotein G. Spikes do not cover the planar end of the virion (virus particle). Beneath the envelope is the membrane or matrix (M) protein layer which may be invaginated at the planar end. The core of the virion consists of helically arranged ribonucleoprotein.
Genome
The genome is unsegmented linear antisense RNA. Also present in the nucleocapsid are RNA dependent RNA transcriptase and some structural proteins.
Life cycle
From the source of wound of entry, the rabies virus travels quickly along the neural pathways to the central nervous system. There the virus further spreads to other organs. The saliva glands located in the tissues of the mouth and cheeks, receive high concentrations and thus allowing for it to further transmitted. The rabies virus typically causes fatality within two days to five years.[3][4]
Prevention
Rabies can be prevented by vaccination, both in humans and other animals. Virtually every infection with rabies resulted in death, until Louis Pasteur and Emile Roux developed the first rabies vaccination in 1885. This vaccine was first used on a human on July 6, 1885 – nine-year old boy Joseph Meister (1876–1940) had been mauled by a rabid dog.[5]
Their vaccine consisted of a sample of the virus harvested from infected (and necessarily dead) rabbits, which was weakened by allowing it to dry for 5 to 10 days. Similar nerve tissue-derived vaccines are still used now in some countries, and while they are much cheaper than modern cell culture vaccines, they are not as effective and carry a certain risk of neurological complications.
The human diploid cell rabies vaccine (H.D.C.V.) was started in 1967. Human diploid cell rabies vaccines are made using the attenuated Pitman-Moore L503 strain of the virus. Human diploid cell rabies vaccines have been given to more than 1.5 million humans as of 2006. Newer and less expensive purified chicken embryo cell vaccine, and purified Vero cell rabies vaccine are now available. The purified Vero cell rabies vaccine uses the attenuated Wistar strain of the rabies virus, and uses the Vero cell line as its host.
Some recent works have shown that during lethal rabies infection the blood-brain barrier (BBB) does not allow anti-viral immune cells to enter the brain, the primary site of rabies virus replication.[6] This aspect contributes to the pathogenicity of the virus and artificially increasing BBB permeability promotes viral clearance.[7] Opening the BBB during rabies infection has been suggested as a possible novel approach to treat the disease.
Post-exposure prophylaxis
Treatment after exposure, known as post-exposure prophylaxis or “P.E.P.”, is highly successful preventing the disease if administered promptly, within six days after infection and consists of over a 28 day period. Thoroughly washing the wound as soon as possible with soap and water for approximately five minutes is very effective at reducing the number of viral particles. “If available, a virucidal antiseptic such as povidone-iodine, iodine tincture, aqueous iodine solution or alcohol (ethanol) should be applied after washing.”[8] Exposed mucous membranes such as eyes, nose or mouth should be flushed well with water. In the United States, patients receive one dose of immunoglobulin and five doses of rabies vaccine over a twenty-eight day period. One-half the dose of immunoglobulin is injected in the region of the bite, if possible, with the remainder injected intramuscularly away from the bite. This is much less painful compared with administering immunoglobulin through the abdominal wall with a large needle, as was done in the past. The first dose of rabies vaccine is given as soon as possible after exposure, with additional doses on days three, seven, fourteen, and twenty-eight after the first. Patients that have previously received pre-exposure vaccination do not receive the immunoglobulin, only the post-exposure vaccinations on day 0 and 3. Since the widespread vaccination of domestic dogs and cats and the development of effective human vaccines and immunoglobulin treatments, the number of recorded deaths in the U.S. from rabies has dropped from one hundred or more annually in the early twentieth century, to 1–2 per year, mostly caused by bat bites, which may go unnoticed by the victim and hence untreated.
P.E.P. is effective in treating rabies because the virus must travel from the site of infection through the peripheral nervous system (nerves in the body) before infecting the central nervous system (brain and spinal cord) and glands to cause lethal damage. This travel along the nerves is usually slow enough that vaccine and immunoglobulin can be administered to protect the brain and glands from infection. The amount of time this travel requires is dependent on how far the infected area is from the brain: if the victim is bitten in the face, for example, the time between initial infection and infection of the brain is very short and P.E.P. may not be successful.
Pre-exposure prophylaxis
Currently pre-exposure immunization has been used on domesticated and normal non-human populations. In many jurisdictions, domestic dogs, cats, and ferrets are required to be vaccinated. A pre-exposure vaccination is also available for humans, most commonly given to veterinarians and those traveling to regions where the disease is common, such as India. Most tourists do not need such a vaccination, just those doing substantial non-urban activities. However, should a vaccinated human be bitten by a carrier, failure to receive subsequent post-exposure treatment could be fatal, although post-exposure treatment for a vaccinated human is far less extensive than that which would normally be required by one with no pre-exposure vaccination.
In 1984 researchers at the Wistar Institute developed a recombinant vaccine called V-RG by inserting the glycoprotein gene from rabies into a vaccinia virus.[9] The V-RG vaccine has since been commercialised by Merial under the trademark Raboral. It is harmless to humans and has been shown to be safe for various species of animals that might accidentally encounter it in the wild, including birds (gulls, hawks, and owls).[10]
V-RG has been successfully used in the field in Belgium, France, and the United States to prevent outbreaks of rabies in wildlife. The virus is stable under relatively high temperatures and can be delivered orally, making mass vaccination of wildlife possible by putting it in baits. The plan for immunization of normal populations involves dropping bait containing food wrapped around a small dose of the live virus. The bait would be dropped by helicopter concentrating on areas that have not been infected yet. Just such a strategy of oral immunization of foxes in Europe has already achieved substantial reductions in the incidence of human rabies. A strategy of vaccinating “neighborhood dogs” in Jaipur, India, (combined with a sterilization program) has also resulted in a large reduction in the number of human cases.[11]
Symptoms
The period between infection and the first flu-like symptoms is normally two to twelve weeks, but can be as long as two years. Soon after, the symptoms expand to slight or partial paralysis, cerebral dysfunction, anxiety, insomnia, confusion, agitation, abnormal behavior, paranoia, terror, hallucinations, progressing to delirium.[citation needed] The production of large quantities of saliva and tears coupled with an inability to speak or swallow are typical during the later stages of the disease; this can result in “hydrophobia”, where the victim has difficulty swallowing because the throat and jaw become slowly paralyzed, shows panic when presented with liquids to drink, and cannot quench his or her thirst. The disease itself was also once commonly known as hydrophobia, from this characteristic symptom. The patient “foams at the mouth” because they cannot swallow their own saliva for days and it gathers in the mouth until it overflows.
Death almost invariably results two to ten days after the first symptoms; the few humans who are known to have survived the disease [citation needed] were all left with severe brain damage, with the recent exception of Jeanna Giese (see below). It is neurotropic in nature.
In Rabies: The Facts[12], Kaplan et. al. describe several typical cases, including one of a 23 year-old Englishwoman:
“On June 17, 1981 she was bitten on the ankle by a dog in New Delhi. On August 18, about two months later, she experienced the first prodromal symptoms. She became anxious and depressed, and it became impossible for her to drink more than small sips of liquid. While sleeping, she frequently sat up in bed suddenly, terrified. On August 19, she became confused, hallucinated, and was incontinent of urine. On August 20, she was unable to eat or drink and was taken to the hospital where she hallucinated and screamed in terror. Misdiagnosed as a psychiatric case, she was injected with a tranquilizer and sent home, however she repeatedly woke up screaming in fear and became so wild and agitated that her husband felt he could not deal with her by himself and took her to her mother's house. She remained terrified, hallucinating and screaming in horror throughout the night. She had no water for almost three days. She fell into a coma the next morning, and died on August 23.”
Diagnosis
The differential diagnosis in a case of suspected human rabies may initially include any cause of encephalitis, particularly infection with viruses such as herpesviruses, enteroviruses, and arboviruses (e.g., West Nile virus). The most important viruses to rule out are herpes simplex virus type 1, varicella-zoster virus, and (less commonly) enteroviruses, including coxsackieviruses, echoviruses, polioviruses, and human enteroviruses 68 to 71. A specific diagnosis may be made by a variety of diagnostic techniques, including polymerase chain reaction (PCR) testing of cerebrospinal fluid, viral culture, and serology. In addition, consideration should be given to the local epidemiology of encephalitis caused by arboviruses belonging to several taxonomic groups, including eastern and western equine encephalitis viruses, St. Louis encephalitis virus, Powassan virus, the California encephalitis virus serogroup, and La Crosse virus.
New causes of viral encephalitis are also possible, as was evidenced by the recent outbreak in Malaysia of some 300 cases of encephalitis (mortality rate, 40%) caused by Nipah virus, a newly recognized paramyxovirus.[13] Similarly, well-known viruses may be introduced into new locations, as is illustrated by the recent outbreak of encephalitis due to West Nile virus in the eastern United States.[14] Epidemiologic factors (e.g., season, geographic location, and the patient’s age, travel history, and possible exposure to animal bites, rodents, and ticks) may help direct the diagnostic workup.
Cheaper rabies diagnosis will be possible for low-income settings according to research reported on the Science and Development Network website in 2008. Accurate rabies diagnosis can be done ten times cheaper, according to researchers from the Farcha Veterinary and Livestock Research Laboratory and the Support International Health Centre in N'Djamena, Chad. The scientists evaluated a method using light microscopy, cheaper than the standard tests, and say this could provide better rabies control across Africa.[15]
Treatments
Induced coma
In 2005, the case of Jeanna Giese, a girl of 15 who survived acute, invaccinated rabies was reported, indicating the successful treatment of rabies through induction of a coma.[16] This treatment approach was based on the theory that rabies' detrimental effects were caused by temporary dysfunctions of the brain, and that the induction of a coma, by producing a temporary partial stop in brain function, would protect the brain from damage while the body built up an immune response to the virus. After thirty-one days of isolation and seventy-six days of hospitalisation, she was released from the hospital, having survived rabies.
Rodney Willoughby Jr., the primary care physician in this case published in the April 2007 issue of Scientific American.[17] He notes that subsequent failures of what he calls the Milwaukee protocol did not use the cocktail of drugs used during the treatment of Giese. A point he makes for future research is the relationship of the virus to depletion of biopterin in the brain.
Later attempts to use the same treatment have failed, but in April, 2008, in Cali, Colombia, it was reported (by local newspapers) that an 11-year-old may have recovered successfully after induction of coma [18]. This patient was infected on February 15 when several children were bitten by a cat in Santander de Quilichao, a small town near Cali. However, this claim has not been verified.
Epidemiology
Vectors

Any mammal may become infected with the rabies virus and develop symptoms, including humans. Most animals can be infected by the virus and can transmit the disease to humans. Infected bats, monkeys, raccoons, foxes, skunks, cattle, wolves, dogs or cats provide the greatest risk to humans. Rabies may also spread through exposure to infected domestic farm animals, groundhogs, weasels and other wild carnivores. Rodents (mice, squirrels etc) are seldom infected.
The virus is usually present in the nerves and saliva of a symptomatic rabid animal.[19][20] The route of infection is usually, but not necessarily, by a bite. In many cases the infected animal is exceptionally aggressive, may attack without provocation, and exhibits otherwise uncharacteristic behaviour[3]. Transmission may also occur via an aerosol through mucous membranes; transmission in this form may have happened in people exploring caves populated by rabid bats.
Transmission between humans is extremely rare, although it can happen through transplant surgery (see below for recent cases), or, even more rarely, through bites, kisses or sexual relations.
After a typical human infection by bite, the virus enters the peripheral nervous system. It then travels along the nerves towards the central nervous system. During this phase, the virus cannot be easily detected within the host, and vaccination may still confer cell-mediated immunity to prevent symptomatic rabies. Once the virus reaches the brain, it rapidly causes encephalitis. This is called the “prodromal” phase. At this time, treatment is useless. Then symptoms appear. Rabies may also inflame the spinal cord producing myelitis.
Prevalence
This section needs expansion with: information from the main article. You can help by adding to it. (October 2008) |

Almost all human deaths caused by rabies originate from Asia and Africa. There are an estimated 55,000 human deaths annually from rabies worldwide, with about 31,000 in Asia, and 24,000 in Africa.[21]
One of the sources of recent flourishing of rabies in East Asia is the pet boom. China introduced in the city of Beijing the “one-dog policy” in November 2006 to control the problem.[22] India has been reported as having the highest rate of human rabies in the world [4].
Rabies and animals
This section needs expansion with: information from the main article. You can help by adding to it. (October 2008) |
Rabies is infectious to mammals.
In dogs three stages of rabies are recognized in dogs and other animals. The first stage is a one to three day period characterized by behavioral changes and is known as the prodromal stage. The second stage is the excitative stage, which lasts three to four days. It is this stage that is often known as furious rabies due to the tendency of the affected dog to be hyperreactive to external stimuli and bite at anything near. The third stage is the paralytic stage and is caused by damage to motor neurons. Incoordination is seen due to rear limb paralysis and drooling and difficulty swallowing is caused by paralysis of facial and throat muscles. Death is usually caused by respiratory arrest.[23]
Recent cases
Several recently publicized cases have stemmed from bats, which are known to be a vector for rabies. See the #Rabies vectors section below.
In October 2004 a female brown bear killed one human and injured several others near the city of Braşov in Central Romania. The bear was killed by human hunters and diagnosed with rabies. More than one hundred humans were vaccinated afterwards.
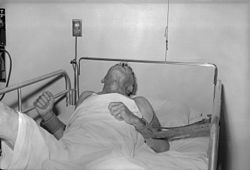
Rabies is known to have been transmitted between humans by transplant surgery on rare occasions.
Infections by corneal transplant have been reported in Thailand (two cases), India (two cases), Iran (two cases),[24] the United States (one case), and France (also a single case).[25] Details of two further cases of infection resulting from corneal transplants were described in 1996.
In June 2004, three organ recipients died in the United States from rabies transmitted in the transplanted kidneys and liver of an infected donor from Texarkana.[26] There were bats near the donor's home, and the donor had told others that he had been bitten.[27] The donor is now reported to have died of a cerebral hemorrhage, the culmination of an unidentified neurological disorder, although recipients are said to have been told the cause of death had been a car crash. Marijuana and cocaine were found in the donor's urine at the time of his death, according to a report in The New England Journal of Medicine.[28]
"[The surgeons] thought he had suffered a fatal crack-cocaine overdose, which can produce symptoms similar to those of rabies. ‘We had an explanation for his condition,’ says Dr. Goran Klintmalm, a surgeon who oversees transplantation at Baylor University Medical Center, where the transplants occurred. ‘He’d recently smoked crack cocaine. He’d hemorrhaged around the brain. He’d died. That was all we needed to know.’ Because of doctor-patient confidentiality rules, doctors involved with this case would not talk about it on the record, but a few did say that if no cocaine was found in the donor’s blood, the E.R. doctors might have investigated his symptoms more aggressively instead of assuming he had overdosed. (Because no autopsy was done, doctors have not been able to establish whether the rabies or the drugs actually killed him.)"[29]
In February 2005, three German patients in Mainz and Heidelberg were diagnosed with rabies after receiving various organs and cornea transplants from a female donor. Two of the infected people died. Three other patients who received organs from the woman have not yet shown rabies symptoms. The 26 year old donor had died of heart failure in December 2004 after consuming cocaine and ecstasy. In October 2004, she had visited India, one of the countries worst affected by rabies worldwide. Dozens of medical staff were vaccinated against rabies in the two hospitals as a precautionary measure. Associated Press reports that "Donated organs are never tested for rabies. The strain detected in the victims’ bodies is one commonly found in bats, health officials said." According to CNN "Rabies tests are not routine donor screening tests, Virginia McBride, public health organ donation specialist with the Health Resources and Services Administration, said. The number of tests is limited because doctors have only about six hours from the time a patient is declared brain-dead until the transplantation must begin for the organs to maintain viability."
See also
References
- ^ a b c d e Mitchell RS, Kumar V, Robbins SL, Abbas AK, Fausto N (2007). Robbins basic pathology. Saunders/Elsevier. ISBN 1-4160-2973-7.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ "Recovery of a patient from clinical rabies--Wisconsin, 2004". MMWR. Morbidity and mortality weekly report. 53 (50): 1171–3. 2004. PMID 15614231.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Baron, Samuel. "Life cycle of rabies". The University of Texas Medical Branch at Galveston. Retrieved 2008-10-10.
{{cite web}}: Cite has empty unknown parameters:|month=and|coauthors=(help) - ^ "Rabies". University of Northern British Columbia. Retrieved 2008-10-10.
{{cite web}}: Cite has empty unknown parameters:|month=and|coauthors=(help) - ^ Geison GL (1978). "Pastuer's work on rabies: Reexamining the ethical issues diagnosis for developing countries". Hastings Center Report (April): 26-.
- ^ Roy A, Phares TW, Koprowski H, Hooper DC (2007). "Failure to open the blood-brain barrier and deliver immune effectors to central nervous system tissues leads to the lethal outcome of silver-haired bat rabies virus infection". J. Virol. 81 (3): 1110–8. doi:10.1128/JVI.01964-06. PMID 17108029.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Roy A, Hooper DC (2007). "Lethal silver-haired bat rabies virus infection can be prevented by opening the blood-brain barrier". J. Virol. 81 (15): 7993–8. doi:10.1128/JVI.00710-07. PMID 17507463.
- ^ Rabies & Australian bat lyssavirus information sheet http://www.health.vic.gov.au/ideas/bluebook/rabies_info
- ^ Wiktor TJ, Macfarlan RI, Reagan KJ, Dietzschold B, Curtis PJ, Wunner WH, Kieny MP, Lathe R, Lecocq JP, Mackett M (1984). "Protection from rabies by a vaccinia virus recombinant containing the rabies virus glycoprotein gene". Proc. Natl. Acad. Sci. U.S.A. 81 (22): 7194–8. doi:10.1073/pnas.81.22.7194. PMID 6095272.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Artois M, Charlton KM, Tolson ND, Casey GA, Knowles MK, Campbell JB (1990). "Vaccinia recombinant virus expressing the rabies virus glycoprotein: safety and efficacy trials in Canadian wildlife". Can. J. Vet. Res. 54 (4): 504–7. PMID 2249183.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Reece JF, Chawla SK. (2006). "Control of rabies in Jaipur, India, by the sterilisation and vaccination of neighbourhood dogs". Vet Rec. 159: 379–83.
- ^ Warrell, D. A.; Kaplan, Colin; Turner, G. S. (1986). Rabies: the facts. Oxford [Oxfordshire]: Oxford University Press. pp. pp. 43-5. ISBN 0-19-261441-X.
{{cite book}}:|pages=has extra text (help)CS1 maint: multiple names: authors list (link) - ^ Taylor DH, Straw BE, Zimmerman JL, D'Allaire S (2006). Diseases of swine. Oxford: Blackwell publishing. ISBN 0-8138-1703-X. Retrieved 2008-10-05.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Minagar, Alireza (2005). Inflammatory Disorders Of The Nervous System: Pathogenesis, Immunology, and Clinical Management. Humana Press. ISBN 1588294242.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Dürr S, Naïssengar S, Mindekem R; et al. (2008). "Rabies diagnosis for developing countries". PLoS neglected tropical diseases. 2 (3): e206. doi:10.1371/journal.pntd.0000206. PMC 2268742. PMID 18365035.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Willoughby RE, Tieves KS, Hoffman GM, Ghanayem NS, Amlie-Lefond CM, Schwabe MJ, Chusid MJ, Rupprecht CE (2005). "Survival after treatment of rabies with induction of coma". N. Engl. J. Med. 352 (24): 2508–14. doi:10.1056/NEJMoa050382. PMID 15958806.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rodney E. Willoughby, Jr., “A Cure for Rabies?” Scientific American, V. 256, No. 4, April 2007, p. 95 (online link)
- ^ El Tiempo Nación Cali, “Nuevos síntomas dan aliento sobre recuperación de niño caucano contagiado por rabia,” April 10th 2008([1])
- ^ The Merck Manual, Eleventh Edition (1983), p. 183
- ^ The Merck manual of Medical Information. Second Home Edition, (2003), p. 484.
- ^ “Rabies” (2006) World Health Organisation. [2]
- ^ The Toronto Star “China cracks down on rabid dog menace”
- ^ Ettinger, Stephen J.;Feldman, Edward C. (1995). Textbook of Veterinary Internal Medicine (4th ed. ed.). W.B. Saunders Company. ISBN 0-7216-6795-3.
{{cite book}}:|edition=has extra text (help)CS1 maint: multiple names: authors list (link) - ^ Javadi MA, Fayaz A, Mirdehghan SA, Ainollahi B (1996). "Transmission of rabies by corneal graft". Cornea. 15 (4): 431–3. doi:10.1097/00003226-199607000-00014. PMID 8776570.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ CDC (1980). "Human-to-human transmission of rabies via a corneal transplant -- France". MMWR. 29: 25–6.
- ^ "Investigation of rabies infections in organ donor and transplant recipients--Alabama, Arkansas, Oklahoma, and Texas, 2004". MMWR Morb Mortal Wkly Rep. 53 (26): 586–9. 2004. PMID 15241303.
- ^ "Update: investigation of rabies infections in organ donor and transplant recipients--Alabama, Arkansas, Oklahoma, and Texas, 2004". MMWR Morb Mortal Wkly Rep. 53 (27): 615–6. 2004. PMID 15254455.
- ^ Srinivasan A, Burt EC, Kuehnert MJ, Rupprecht C, Sutker WL, Ksiazek TG, Paddock CD, Guarner J, Shieh WJ, Goldsmith C, Hanlon CA, Zoretic J, Fischbach B, Niezgoda M, El-Feky WH, Orciari L, Sanchez EQ, Likos A, Klintmalm GB, Cardo D, LeDuc J, Chamberland ME, Jernigan DB, Zaki SR (2005). "Transmission of rabies virus from an organ donor to four transplant recipients". N Engl J Med. 352 (11): 1103–11. doi:10.1056/NEJMoa043018. PMID 15784663.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Reynolds G (2005). "Will Any Organ Do?". The New York Times Magazine (10 July): –.
Further reading
- Waterman JA (1959). "The history of the outbreak of paralytic rabies in Trinidad transmitted by bats to human beings and the lower animals from 1925". Caribb Med J. 21: 1–6. PMID 13843069.
- Fleming, Theodore H. (2003). A bat man in the tropics: chasing El Duende. Berkeley: University of California Press. ISBN 0-520-23606-8.
- Warrell, D. A.; Kaplan, Colin; Turner, G. S. (1986). Rabies: the facts. Oxford [Oxfordshire]: Oxford University Press. ISBN 0-19-261441-X.
{{cite book}}: CS1 maint: multiple names: authors list (link)
