Sulfur dioxide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Sulfur dioxide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | SO 2 | |||||||||||||||
| Brief description |
colorless, pungent smelling, poisonous gas |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 64.06 g mol −1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| Melting point |
−75.5 ° C |
|||||||||||||||
| boiling point |
−10.05 ° C |
|||||||||||||||
| Vapor pressure |
0.3271 M Pa (20 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Dipole moment | ||||||||||||||||
| Refractive index |
1,000686 (0 ° C, 101.325 kPa) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
|
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Sulfur dioxide , SO 2 , is the anhydride of the sulphurous acid H 2 SO 3 . Sulfur dioxide is a colorless, mucous membrane-irritating, pungent smelling and sour-tasting, poisonous gas. It is very well (physically) soluble in water and forms sulfurous acid to a very small extent with water. It arises, among other things, from the combustion of sulfur-containing fossil fuels such as coal or petroleum products , which contain up to 4 percent sulfur . As a result, it contributes significantly to air pollution . It is the reason for acid rain , whereby the sulfur dioxide is first oxidized by oxygen to sulfur trioxide and then reacted with water to form sulfuric acid (H 2 SO 4 ). There are various methods of flue gas desulphurisation to prevent the entry of sulfur dioxide . In addition, sulfur dioxide is found in the vicinity of high-temperature areas and active volcanoes .
Manufacturing
Sulfur dioxide can be produced by several processes:
- by burning sulfur or hydrogen sulfide
- by roasting sulphidic ores, e.g. B. of pyrite :
- from sulfites through stronger acids
properties
Physical Properties
Sulfur dioxide has a relative gas density of 2.26 (density ratio to dry air at the same temperature and pressure ) and a density of the liquid phase at the boiling point of 1.458 kg / l. The gas density under normal conditions (0 ° C, 1013 mbar) is 2.9285 kg · m −3 , at a temperature of 15 ° C and a pressure of 1 bar, however, 2.728 kg · m −3 . The critical temperature is 157.5 ° C, the critical pressure is 78.8 bar and the critical density is 0.525 g · cm −3 . At the triple point there is a temperature of -75.5 ° C and a pressure of 16.75 bar.
Chemical properties
Sulfur dioxide is a colorless, pungent smelling and corrosive gas. It dissolves well in water , creating a weakly acidic solution. Sulfur dioxide also acts as a reducing agent .
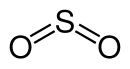
The sulfur dioxide molecule can be described by two mesomeric boundary structures:
The two σ bonds (two S – O bonds) and the lone pair of electrons on the S atom are formed by the s and the two p orbitals of the S atom. The π bond is delocalized over the entire molecule (multi-center π bond).
Molecular geometry
According to the VSEPR model , the sulfur dioxide molecule is built at an angle. This results in a bond angle (O – S – O) of 119.5 °. The two S – O bonds are of the same length with a bond length of 143 pm and are therefore very short.
As a molecular symmetry, sulfur dioxide has the point group C 2v .
use
Liquid sulfur dioxide dissolves numerous substances and has therefore established itself as a valuable aprotic-polar solvent .
In the food industry , sulfur dioxide is used as a preservative , antioxidant and disinfectant , especially for dried fruits, potato dishes, fruit juices, jams and wine . Wine and beer kegs are used for disinfection prior to use by treatment with SO 2 gas sulphured .
Sulfur dioxide destroys the vitamin B1 ; There are also indications of the destruction of B12 vitamins in laboratory tests . In the EU , it is also approved as a food additive with the number E 220 for " organic " products. It is also used to produce sulfuryl chloride SO 2 Cl 2 and thionyl chloride SOCl 2 . In sulfochlorination , it is used to produce surfactants .
In addition, sulfur dioxide is an important starting material for the production of sulfur trioxide , in order to subsequently use concentrated sulfuric acid e.g. B. to produce with the contact method .
Sulfur dioxide is also used in the manufacture of many chemicals, medicines and dyes and in the bleaching of paper and textiles. It fades ink.
It is also used as a protective gas , for example to prevent molten metal in the foundry from oxidizing.
storage
In industry, sulfur dioxide is mostly obtained from pressurized gas cylinders and is often stored for temperature control in the production environment, i.e. indoors, often right next to thermal processing systems . As part of the risk assessment, it must be determined for the storage of sulfur dioxide in accordance with Section 5 of the Occupational Safety and Health Act and Section 6 of the GefStoffV whether the storage of hazardous substances could pose a risk to employees or other people.
For the storage of sulfur dioxide, the following regulations apply in particular (in Germany):
- TRGS 510 Storage of hazardous substances in portable containers
- TRBS 3145 / TRGS 745 Transportable pressurized gas containers - filling, provision, internal transport, emptying
- TRBS 3146 / TRGS 726 Fixed pressure systems for gases
To protect the health of employees and to meet the requirements of the Occupational Safety and Health Act, sulfur dioxide bottles must therefore be kept in a suitable storage facility. The appropriately equipped safety gas cylinder cabinet, whose special equipment for sulfur dioxide storage consists of the following essential components, is suitable for this:
- The safety cabinet itself, for one or more sulfur dioxide bottle (s) and a nitrogen gas bottle. Often two bottles of sulfur dioxide are provided for operation and automatic switching. One bottle is in the cabinet for pre-tempering, the gas is taken from the second bottle for use. The nitrogen gas bottle is used to supply the flushing device for safe bottle changes. The safety cabinet is designed as a fire-retardant cabinet, as the pressurized gas can explode when heated and cause severe burns to the skin and serious damage to eyes. The cabinet should be lockable as the safety data sheet for sulfur dioxide specifies “P405 - Keep locked up”.
- Sulfur dioxide pressure control station made of stainless steel with automatic switchover, for uninterrupted media supply. The sulfur dioxide gas bottles are connected with a corrugated stainless steel hose. The fittings used must be made of stainless steel, as sulfur dioxide is converted into sulfuric acid (H 2 SO 4 ) with humidity .
- An automatic shut-off solenoid valve enables the sulfur dioxide process line to be shut off to the point of use in the event of an emergency stop or a gas alarm.
- The gas warning sensor in the safety cabinet, if necessary with additional gas warning sensors in the vicinity of the points of use, generates the gas alarm with an optical and acoustic signal.
- External ventilation with fan for manual operation by the user and automatic triggering when the gas warning device is addressed. The ventilation outlet is usually connected to a ventilation system so that the gases do not escape into the production environment.
With this special equipment of the sulfur dioxide cabinet, employees are safely protected from the risk of excessive sulfur dioxide concentration in the air they breathe and from chemical burns in the event of accidents.
Use in concentration camps
In experiments with poison gases that were carried out in the Croatian concentration camp Stara Gradiška , in addition to Zyklon B , sulfur dioxide was also used on Serb, Jewish and Roma women and children.
environmental pollution
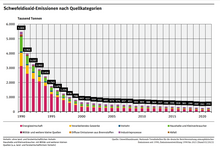
Sulfur dioxide is harmful to humans, animals and plants in high concentrations. The oxidation products lead to “ acid rain ”, which endangers sensitive ecosystems such as forests and lakes and attacks buildings and materials. This also includes the weakening of forest plants , which suffer greater frost damage after extraordinary winters than comparable plants in less polluted areas. The SO 2 - emissions , however, the developed industrial countries have in the last two decades through the use of low-sulfur or sulfur-free fuels and fuels and means of flue gas desulphurization be greatly reduced.
Of all the modes of transport , international shipping makes the highest contribution to emissions. There, the maximum permissible sulfur content in fuel for ships is currently 4.5 percent. However, the IMO has reduced the limit value: it is to be reduced to 3.5 percent by 2012 and to 0.5 percent by 2020. This limit already applies to California coastal waters today. In the Baltic and North Sea there are sulfur emission monitoring areas ( SECA ), in which the limit value is 1.5 percent today. From July 1, 2010, it will be 1 percent and on January 1, 2015, it will be reduced to 0.1 percent.
In 2019, a study by Transport and Environment was published which shows that Carnival's cruise ships alone emitted almost ten times more sulfur oxides along Europe's coasts in 2017 than all 260+ million passenger vehicles in Europe combined. As part of a study, the Max Planck Institute for Meteorology was able to show that the cloud cover in the vicinity of the heavily frequented seaports Rotterdam , Antwerp and Milford Haven is considerably denser than in the surrounding area. Sulfur dioxide and nitrogen oxides act as condensation nuclei and stimulate cloud formation. The increased albedo caused by this cloud cover led to a reduction in solar radiation in the areas below.
Globally, too, sulfur dioxide can contribute to clouding the atmosphere by increasing the aerosol content, for example after strong volcanic eruptions .
safety instructions
A sulfur dioxide concentration above the MAK value can lead to headaches, nausea and drowsiness in humans. In higher concentrations, the gas severely damages the bronchi and lungs.
Long-term exposure to high concentrations of sulfur dioxide can lead to anemia by destroying the B12 vitamin, which is important for blood formation .
Individual evidence
- ↑ a b c d e f g Entry on sulfur dioxide in the GESTIS substance database of the IFA , accessed on May 4, 2020(JavaScript required) .
- ↑ a b c Safety data sheet sulfur dioxide (PDF, p. 8/15.) Pangas.ch, accessed on January 12, 2016.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Dipole Moments, pp. 9-52.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Index of Refraction of Gases, pp. 10-254.
- ↑ Entry on Sulfur dioxide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Schweizerische Unfallversicherungsanstalt (Suva): Limits - Current MAK and BAT values (search for 7446-09-5 or sulfur dioxide ), accessed on May 16, 2020.
- ↑ Entry on sulfur dioxide in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 .
- ^ HU Schmincke: Vulcanism. Knowledge Buchgesellschaft, Darmstadt 2000, p. 224 ff.
- ^ A b c Erwin Riedel, Christoph Janiak: Inorganische Chemie . 9th edition. Walter de Gruyter GmbH, Berlin 2015, ISBN 978-3-11-035526-0 , p. 470 .
- ↑ Erwin Riedel, Christoph Janiak: Inorganic Chemistry . 9th edition. Walter de Gruyter GmbH, Berlin 2015, ISBN 978-3-11-035526-0 , p. 238 .
- ↑ Grillo-Werke AG | SULFUR DIOXIDE LIQUID . ( grillo.de [accessed on November 9, 2018]).
- ↑ H.-D. Belitz, W. Grosch: Food Chemistry. Springer Verlag, Berlin / Heidelberg 1999.
- ↑ Matthias Bünck: casting properties. In: Andreas Bührig-Polaczek, Walter Michaeli, Günter Spur (eds.): Handbuch Spanen. Hanser, Munich 2014, p. 36.
- ↑ a b SO2 cabinet for health protection - LT gas technology . In: LT Gas Technology . October 26, 2016 ( lt-gasetechnik.de [accessed April 12, 2017]).
- ↑ Michele Frucht Levy: "The Last Bullet for the Last Serb" - The Ustasa Genocide against Serbs 1941-1945. In: David M. Crowe (Ed.): Crimes of State Past and Present. Routledge, 2011, ISBN 978-0-415-57788-5 , p. 71. ( limited preview in Google book search)
- ^ Theodor Keller: Frost damage as a result of latent pollution damage. In: Dust - cleanliness. Air . 38, No. 1, 1978, ISSN 0949-8036 , pp. 24-26.
- ↑ wiwo.de: New drive for ships ( Memento of the original from March 21, 2008 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. .
- ↑ IMO: The 2008 amendments-revised Annex VI .
- ↑ Institute of Shipping Economics and Logistics: SECA study final report (PDF; 1.8 MB), September 2010.
- ↑ One corporation to pollute them all. In: transportenvironment.org. June 4, 2019, accessed June 16, 2019 .
- ↑ Massive exhaust emissions in ports from cruise ships. In: nabu.de . June 5, 2019, accessed June 16, 2019 .
- ↑ planet-erde.de: Thick air by the sea
- ↑ Zusatzstoffe-online.de: sulfur dioxide E220