Fluorcalcioroméit
| Fluorcalcioroméit | |
|---|---|
| Pseudomorphosis of lanceolate fluoroccalcioroméite crystals based on antimonite and / or bournonite on barite from the “Corta Santa Matilde” belonging to the “Grupo Minero Berja” in the municipality of Las Herrerías in the Sierra Almagrera, Cuevas de Almanzora , Almería , Andalusia , Spain (size: 7.4 × 5.5 × 5.2 cm) | |
| General and classification | |
| other names |
|
| chemical formula |
|
|
Mineral class (and possibly department) |
Oxides and hydroxides |
|
System no. to Strunz and to Dana |
4.DH.15 ( 8th edition :?) ? |
| Crystallographic Data | |
| Crystal system | cubic |
| Crystal class ; symbol | cubic hexakisoctahedral; 4 / m 3 2 / m |
| Space group | Fd 3 m (No. 227) |
| Lattice parameters | a = 10.2987 Å |
| Formula units | Z = 8 |
| Frequent crystal faces | {111} |
| Twinning | no |
| Physical Properties | |
| Mohs hardness | ≈ 5.5 |
| Density (g / cm 3 ) | 5.113 (calculated) |
| Cleavage | no |
| Break ; Tenacity | clamshell; brittle |
| colour | yellow to orange |
| Line color | White |
| transparency | translucent |
| shine | Glass to resin gloss |
| Crystal optics | |
| Refractive index | n = 1.826 |
| Optical character | isotropic |
Fluorcalcioroméit is a very rare mineral from the mineral class of oxides and hydroxides . It crystallizes in the cubic crystal system with the composition (Ca, Na) 2 Sb 5+ 2 (O, OH) 6 F, so it is a calcium - sodium - antimonate , the Y position of which is mainly occupied by fluorine ions.
Fluorcalcioroméit occurs at its type locality in the form of idiomorphic , octahedral crystals with a maximum size of 1 mm, which are closely associated with braunite , hematite , calcite , quartz and rarely also wallkilldellite (Mn) .
The type locality of Fluorcalcioroméits is in Triassic Plattformcarbonaten seated manganese - iron - deposit of the mine "Starlera" ( coordinates of pit Starlera ) in Ferrera GR in Val Ferrera , Hinterrhein , Grisons , Switzerland . The "Starlera" pit is already the type locality for manganese solder armyerite .
Etymology and history
Chemical analyzes that corresponded to a fluorine calculus were already known before the turn of the millennium. These include analyzes from the “Fianel” mine near Ausserferrera and from the “Starlera” mine near Ferrera GR , both in Val Ferrera, Hinterrheintal, Graubünden, Switzerland, the granite pegmatites from Prašivá in Slovakia and again from the “Starlera” mine. In 2010, the IMA presented a new nomenclature for the minerals of the newly defined pyrochlore upper group (pyrochlore supergroup). It stipulated that the Ca-Sb-F-dominant member of this upper group is to be referred to as the fluorcalcioroméit.
After determining its physical, chemical and structural properties, a mineral from the “Starlera” mine turned out to be identical to Fluorcalcioroméit in the sense of the new nomenclature of the pyrochlore upper group. The new mineral was presented to the International Mineralogical Association (IMA), which recognized it in 2012 under the tentative designation IMA 2012-093 . The first scientific description of this mineral took place in 2013 by a Brazilian-Italian research team with Daniel Atencio , Marco E. Ciriotti and Marcello B. Andrade in the English science magazine Mineralogical Magazine . The authors named the mineral in accordance with the nomenclature of the pyrochlore upper group due to its chemical composition with an A position dominated by calcium , a B position dominated by Sb and a Y position dominated by fluorine as Fluorcalcioroméit ( English Fluorcalcioroméite ).
The type material for Fluorcalcioroméit is kept under the catalog number M / 15925 (holotype) in the collection of the “Museo Regionale di Scienze Naturali, Sezione di Mineralogia, Petrografia e Geologia” in Turin , Italy . Cotyp stages are in the collection of the RRUFF project (catalog number R120140), and in the collection of the “Museu de Geociências” at the “Instituto de Geociências”, Universidade de São Paulo in São Paulo , State of São Paulo , Brazil (catalog number DR745 ).
Roméit was a mineral named in 1841 by Augustin Alexis Damour in honor of Jean-Baptiste Romé de L'Isle , French mineralogist and one of the founders of crystallography, which was discredited when the nomenclature of the pyrochlore upper group was redefined in 2010 because it hide behind its composition the new minerals fluoronatroroméite, fluorcalcioroméite and oxycalcioroméite. He is also the namesake for the Roméit sub-group within the pyrochlore upper group. When the Atopit designated Roméit -Varietät then possibly also to Fluorcalcioroméit.
classification
The current classification of the International Mineralogical Association (IMA) counts the Fluorcalcioroméit to the pyrochlore upper group with the general formula A 2– m B 2 X 6– w Y 1– n , in which A , B , X and Y different positions in the structure the minerals of the pyrochlore upper group with A = Na, Ca, Sr, Pb 2+ , Sn 2+ , Sb 3+ , Y, U, □, or H 2 O; B = Ta 5+ , Nb 5+ , Ti 4+ , Sb 5+ , W 6+ , Al 3+, or Mg 2+ ; X = O, OH or F and Y = OH - , F, O, □, H 2 O or very large (>> 1.0 Å) monovalent cations such as K, Cs or Rb. To pyrochlore supergroup include not only Fluorcalcioroméit still Fluorcalciomikrolith , Fluornatromikrolith , Hydrokenomikrolith , Hydroxycalciomikrolith , Hydroxykenomikrolith , Kenoplumbomikrolith , Oxynatromikrolith , Oxystannomikrolith , Oxystibiomikrolith , Cesiokenopyrochlor , Fluorcalciopyrochlor , Fluornatropyrochlor , Hydrokenopyrochlor , Hydropyrochlor , Hydroxycalciopyrochlor , Hydroxykenopyrochlor , Hydroxymanganopyrochlor , Hydroxynatropyrochlor , Oxycalciopyrochlor , Hydroxycalcioroméit , Hydroxyferroroméit , Oxycalcioroméit , Oxyplumboroméite , Hydrokenoelsmoreit , Hydroxykenoelsmoreit , Fluornatrocoulsellit and Hydrokenoralstonit . Fluorcalcioroméit forms together with Hydroxycalcioroméit, Hydroxyferroroméit, Oxycalcioroméit and Oxyplumboroméite within the pyrochlore upper group Roméitgruppe .
The 8th edition of the mineral classification according to Strunz , which is now outdated, but still in use in some cases, does not yet list the fluoroccalcioroméit.
The 9th edition of Strunz's mineral systematics , which has been in effect since 2001 and is used by the International Mineralogical Association (IMA), classifies the Fluorcalcioroméit in the department of “Oxides with the molar ratio of metal: oxygen = 1: 2 and comparable”. This is further subdivided according to the relative size of the cations involved and the crystal structure, so that the mineral is classified according to its composition and structure in the subsection “With large (± medium-sized) cations; Layers of edge-linked octahedra ”can be found, where together with all representatives of the pyrochlore, microlith, Betafit, Roméit and Elsmoreit groups, the pyrochlore supergroup with the system no. 4.DH.15 forms. Fluorcalcioroméit is composed mi Fluornatroroméit , Hydroxycalcioroméit (formerly lewisite ) Oxycalcioroméit , Oxyplumboroméite , Bismutostibiconit (Q), Monimolit (Q), Partzit (Q), Stetefeldtit (Q), Stibiconit to find (Q) in the Roméitgruppe.
The systematics of minerals according to Dana , which is mainly used in the English-speaking world, also does not yet know the fluorcalcioroméit.
Chemism
Thirteen microprobe analyzes on fluoroccalcioroméite grains from the type locality yielded mean values of 4.11% Na 2 O; 15.41% CaO; 0.54% MnO; 0.01 CuO; 0.01 ZnO; 0.02% PbO; 0.10% Al 2 O 3 ; 0.50% FeO; 0.07% Y 2 O 3 ; 0.04% SiO 2 ; 0.01% TiO 2 ; 0.01% UO 2 ; 76.18% Sb 2 O 3 ; 0.78% WO 3 ; 2.79% F; 0.59% H 2 O; (O ≡ F) -1.17%; Total = 100.00%. On the basis of two cations on the B position per formula unit, the empirical formula (Ca 1.16 Na 0.56 ◻ 0.22 Fe 2+ 0.03 Mn 2+ 0.03 ) Σ = 2.00 ( Sb 5+ 1.98 Al 0.01 W 0.01 ) Σ = 2.00 O 6 [F 0.62 (OH) 0.28 O 0.06 ◻ 0.04 ] Σ = 1.00 calculated, the simplified to (Ca, Na) 2 Sb 5+ 2 O 6 (F, OH). The charge-balanced end link variant of this formula is given as (Ca 1.50 ◻ 0.50 ) Sb 5+ 2 O 6 F.
The only side Fluorcalcioroméit minerals with the combination of elements Ca - Na - Sb - O - F are not described as a mineral Fluornatroroméit , (Na, Ca) 2 Sb 2 (O, OH) 6 F, and the phases in question Atopit , (Ca , Na) 2 Sb 2 (O, F, OH) 7 , and mauzeliite , (Pb, Ca, Na) 2 (Sb, Ti) 2 (O, OH, F) 7 .
Within the pyrochlore upper group there are theoretically a multitude of substitution possibilities due to the four different positions to be occupied. Fluorcalcioroméit is the F-dominant analogue of the O-dominated oxycalcioroméite and to the OH-dominated hydroxycalcioroméit. Across all subgroups, Fluorcalcioroméit is the Sb 5+ -dominant analogue of the Ta-dominated Fluorcalciomikrolith and the Nb-dominated Fluorcalciopyrochlor.
Crystal structure
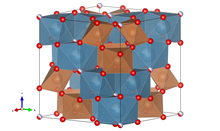
Fluorcalcioroméit crystallizes in the cubic crystal system in the space group Fd 3 m (space group no. 227) with the lattice parameter a = 10.2987 Å and eight formula units per unit cell .
The crystal structure of the Fluorcalcioroméits (compare with the adjacent structural drawing) can be used as three-dimensional, typical for representatives of the pyrochlore supergroup framework of corner-sharing B O 6 - octahedra are described, wherein in the interstices of this scaffold, the A cations and the oxygen ions are sitting. The eight-coordinate A position (16 d ) is mainly occupied by Ca and Na. The octahedral coordinated B position ( 16c ) is only occupied by Sb 5+ . The X - (48 f ) and Y positions (8 b ) are four-coordinate anion positions and are occupied by O and OH (48 f ) and F (8 b ). The oxygen environment around the B cations is roughly octahedral, while the A cations are located in distorted cubes.
Fluorcalcioroméit is isotypic (isostructural) to all other representatives of the pyrochlore upper group which crystallize in space group Fd 3 m (space group no. 227) .
properties
morphology
Fluorcalcioroméit forms idiomorphic octahedral, never twinned crystals {111} from 0.1 mm to 1 mm in size at its type locality. In addition, pseudomorphoses of fluorcalcioroméite after lanceolate antimonite and / or bournonite crystals are known (compare the picture in the info box).
physical and chemical properties
The crystals of the Fluorcalcioroméit are yellow to orange in color, while their line color is indicated as white. The surfaces of the translucent Fluorcalcioroméit show a glass-like to resinous gloss , which agrees very well with the moderately high value for the refraction of light (n = 1.826). Due to its membership in the cubic crystal system, fluorine calcioroméit has no birefringence and is optically completely isotropic.
Fluorcalcioroméit is neither cleavable nor divisible, but due to its brittleness it breaks like quartz , with the fracture surfaces being formed like a shell. With a Mohs hardness of ≈ 5.5, the mineral is one of the medium-hard minerals and, like the reference minerals apatite (hardness 5), can still be scratched with a pocket knife or, like Orthoclase (hardness 6), only scratched with a steel file. The calculated density for Fluorcalcioroméite is 5.113 g / cm³. Fluorcalcioroméit is neither in the long wavelength even in the short wavelength UV light , a fluorescent .
Education and Locations
The type locality of the Fluorcalcioroméit is the small iron-manganese deposit "Starlera" located at an altitude of 2,400 m above sea level near Ferrera GR in Val Ferrera , Hinterrhein , Graubünden , Switzerland , from which 145 tons of manganese ore were mined between 1918 and 1920 are. The deposit consists of retrograde metamorphosed , syngenetic - exhalative manganese ores; the ores of the Val Ferrera in carbonates have a complex geochemical association with Fe – Mn– (Ba, V, As, Sb, Be, W, REE), which is mainly due to their syngeneic, exhalative formation and their location over a granite-rich continental Basement. Paragenesis minerals of the Fluorcalcioroméits in the "Starlera" mine are Braunite , Hematite , Calcite , Quartz and, rarely, Wallkilldellite (Mn) .
As a rare mineral formation, the Fluorcalcioroméit could so far (as of 2018) only be described from about ten sites worldwide.
In addition to the type locality, the following sites are known:
- Metamorphic manganese mineralization at the Obernberger Tribulaun , Obernbergtal , Wipptal , Stubai Alps , North Tyrol , Austria
- Granite pegmatite dikes in the Sopotnická Dolina valley near Brusno , Okres Banská Bystrica , Banskobystrický kraj , Slovakia
- the Corta (open pit) “Santa Matilde” belonging to the “Grupo Minero Berja” in the municipality of Las Herrerías in the Sierra Almagrera, Cuevas de Almanzora (Cuevas de Vera), Almería , Andalusia , Spain
- the metamorphic Fe-Mn deposit Långban , Filipstad municipality , Värmland County or the historic Värmland province in central Sweden
- the "Fianel" mine not far from the Ausserferrera fraction of the municipality of Ferrera GR in the south of the former Hinterrhein district of the canton of Graubünden , Switzerland
- "Schmorrasgrat-Süd" between the Ferreratal (Mulegn-Tal) and the Oberhalbstein (Schmorras-Tal) near Ausserferrera, Val Ferrera, Hinterrhein-Tal, Graubünden, Switzerland ("Schmorrasgrat-Süd" is the southern segment of the ridge between Fuorcla and Cotschna Piz Alv)
- the "Alp Tanatz" near Splügen GR , a part of the municipality of Rheinwald GR , Viamala region , Hinterrhein Valley, Graubünden, Switzerland
Locations for fluorcalcioroméit in Germany and Austria are therefore unknown.
use
Fluorcalcioroméit is because of its rarity without any practical meaning and only interesting for mineral collectors.
See also
literature
- Daniel Atencio, Marco E. Ciriotti Marcello B. Andrade: Fluorcalcioroméite, (Ca, Na) 2 Sb 5+ 2 (O, OH) 6 F, a new roméite-group mineral from Starlera mine, Ferrera, Grischun, Switzerland: Description and crystal structure . In: Mineralogical Magazine . tape 77 , no. 4 , 2012, p. 467–473 , doi : 10.1180 / minmag.2013.077.4.06 (English, researchgate.net [PDF; 939 kB ; accessed on October 26, 2018]).
Web links
- Mineral Atlas: Fluorcalcioroméit (Wiki)
- Fluorcalcioroméite. In: mindat.org. Hudson Institute of Mineralogy, accessed September 5, 2019 .
- American-Mineralogist-Crystal-Structure-Database - Fluorcalcioroméite. In: rruff.geo.arizona.edu. Retrieved September 5, 2019 .
Individual evidence
- ↑ a b c d Fluorcalcioroméite. In: mindat.org. Hudson Institute of Mineralogy, accessed September 5, 2019 .
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x y z aa from Daniel Atencio, Marco E. Ciriotti Marcello B. Andrade: Fluorcalcioroméite, (Ca, Na) 2 Sb 5+ 2 (O, OH) 6 F, a new roméite-group mineral from Starlera mine, Ferrera, Grischun, Switzerland: Description and crystal structure . In: Mineralogical Magazine . tape 77 , no. 4 , 2012, p. 467–473 , doi : 10.1180 / minmag.2013.077.4.06 (English, researchgate.net [PDF; 939 kB ; accessed on October 26, 2018]).
- ↑ a b Joël Brugger, Sergey V. Krivovichev, Uwe Kolitsch, Nicolas Meisser, Michael Andrut, Stefan Ansermet, Peter C. Burns: Description and crystal structure of manganlotharmeyerite, Ca (Mn 3+ , ◻, Mg) 2 {AsO 4 , [AsO 2 (OH) 2 ]} 2 (OH, H 2 O) 2 from the Starlera Mn deposit, Swiss Alps, and a redefinition of lotharmeyerite . In: The Canadian Mineralogist . tape 40 , no. 4 , 2002, p. 1597–1608 , doi : 10.2113 / gscanmin.40.6.1597 ( rruff.info [PDF; 1.1 MB ; accessed on February 20, 2018]).
- ↑ Joël Brugger, Reto Gieré, Stefan Grasses, Nicolas Meisser: The crystal chemistry of roméite . In: Contributions to Mineralogy and Petrology . tape 127 , 1997, pp. 136-3146 , doi : 10.1007 / s004100050271 (English, researchgate.net [PDF; 1.3 MB ; accessed on October 26, 2018] as Roméite).
- ↑ Pavel Uher, Petr Černý, Ron Chapman, Jozef Határ, Oto Miko: Evolution of Nb, Ta-oxide minerals in the Prašivá granitic pegmatites, Slovakia. II. External hydrothermal Pb, Sb overprint . In: The Canadian Mineralogist . tape 36 , 1998, pp. 535-545 (English, researchgate.net [PDF; 1.4 MB ; accessed on October 26, 2018] as Roméite).
- ↑ Joël Brugger, Reto Gieré: As, Sb, Be and Ce enrichment in minerals from a metamorphosed Fe-Mn deposit, Val Ferrera, Eastern Swiss Alps . In: The Canadian Mineralogist . tape 37 , no. 1 , 1999, p. 37–52 (English, researchgate.net [PDF; 1.8 MB ; accessed on October 26, 2018] as Roméite).
- ↑ a b c d e Daniel Atencio, Marcelo B. Andrade, Andrew G. Christy, Reto Gieré, Pavel M. Kartashov: The Pyrochlore supergroup of minerals: Nomenclature . In: The Canadian Mineralogist . tape 48 , 2010, p. 673–698 , doi : 10.3749 / canmin.48.3.673 (English, rruff.info [PDF; 1,4 MB ; accessed on August 30, 2018]).
- ^ Andrew G. Christy, Daniel Atencio: Clarification of the status of species in the pyrochlore supergroup . In: Mineralogical Magazine . tape 77 , no. 1 , 2013, p. 13–20 , doi : 10.1180 / minmag.2013.077.1.02 (English, cnmnc.main.jp [PDF; 85 kB ; accessed on August 30, 2018]).
- ↑ atopites. In: mindat.org. Hudson Institute of Mineralogy, accessed September 5, 2019 .
- ↑ Cristian Biagioni, Paolo Orlandi, Fabrizio Nestola, Sara Bianchin: Oxycalcioroméite, Ca 2 Sb 2 O 6 O, from Buca della Vena mine, Apuan Alps, Tuscany, Italy: a new member of the pyrochlore supergroup . In: Mineralogical Magazine . tape 77 , 2013, p. 3027–3037 , doi : 10.1180 / minmag.2013.077.7.12 (English).
- ^ Franz Eugen Hussak, George Thurland Prior: Lewisite and zirkelite, two new Brazilian minerals . In: Mineralogical Magazine . tape 11 , 1895, p. 80–88 , doi : 10.1180 / minmag.1895.011.50.05 (English, rruff.info [PDF; 331 kB ; accessed on September 5, 2019]).
- ↑ Marcelo B. Andrade, Daniel Atencio, Aba IC Persiano and Javier Ellena: Fluorcalciomicrolite, (Ca, Na, □) 2 Ta 2 O 6 F, a new microlite-group mineral from Volta Grande pegmatite, Nazareno, Minas Gerais, Brazil . In: Mineralogical Magazine . tape 77 , 2013, p. 2989–2996 , doi : 10.1180 / minmag.2013.077.7.08 (English, researchgate.net [PDF; 494 kB ; accessed on October 26, 2018]).
- ↑ Li Guowu, Yang Guangming, Lu Fude, Xiong Ming, Ge Xiangkun, Pan Baoming, Jeffrey de Fourestier: Fluorcalciopyrochlor, a new mineral species from Bayan Obo, Inner Mongolia, PR China . In: The Canadian Mineralogist . tape 54 , no. 5 , 2016, p. 1285–1291 , doi : 10.3749 / canmin.1500042 (English).
- ↑ Localities for Fluorcalcioroméite. In: mindat.org. Hudson Institute of Mineralogy, accessed September 5, 2019 .
- ↑ a b List of locations for Fluorcalcioroméit in the Mineralienatlas and Mindat (accessed on October 26, 2018)