Light stabilizers
Light protection agents are substances that protect organisms or technical products from the harmful effects of light, especially UV radiation . The protective effect is created by the absorption , reflection or scattering of the UV radiation, which means that less radiation penetrates the body to be protected. For humans, the sun cream is particularly relevant, which protects against skin cancer through its use . In terms of technical products, polymers and lacquers should be mentioned here, which are mixed with light stabilizers, mostly as additives.
UV absorber (UV filter)
UV absorbers (synonymous with “UV filters”) can be found in cosmetics - such as sun creams - to protect the skin or in objects to protect the materials. The concentration of filter substances has a direct influence on the sun protection factor . This effect obeys the principle of light absorption ( Lambert-Beer law ). The amount of UV radiation absorbed is a function of the thickness of the body irradiated (the layer applied) and the concentration of the UV absorber. It is released again as thermal energy.
UV absorbers approved in Europe are listed in the German Cosmetics Ordinance . Since the individual substances usually do not offer protection across the entire UV spectrum, several substances are usually combined, as the effects of different UV absorbers complement each other. The UV absorber serves as a coating and is partially absorbed into the horny layer of the skin.
The effect of the UV absorber is based on the Stokes shift in the case of absorbed UV radiation ; in the case of organic UV absorbers, the Stokes shift occurs due to conjugated double bonds . A distinction is made between UVA, UVB and broadband filters (UVA / UVB absorber) according to their absorption spectrum . Organic UV absorbers are often derivatives of camphor , salicylic acid (e.g. homosalate ) or cinnamic acid . Inorganic UV absorbers are z. B. finely divided titanium dioxide and zinc oxide particles. Since these UV absorbers enable order control via their visibility, they are often used in children's products. An alternative to UV absorbers are textile coverings such as clothing and staying in the shade , e.g. B. under a parasol .
In addition, UV absorbers such as benzotriazoles are also generally used for material protection .
Nanoscale titanium dioxide can be used as an inorganic UV absorber in paints . In contrast to the coarser titanium dioxide used as a pigment , very fine types are transparent and therefore do not cause any clouding of the paint. In contrast, untreated titanium dioxide pigments in particular are the main component of the chalking cycle and thus a main reason for the damage caused by chalking . Iron oxide pigments , on the other hand, only act as UV absorbers, but can not be used in all applications due to their inherent color .
In food packaging may zinc oxide nanoparticles as UV absorbers in use. If zinc oxide nanoparticles are transferred to these foods, consumption can lead to changes in the intestines and a reduction in nutrient absorption .
Types of UV absorbers
Organic UV absorbers:
- 2- (2-Hydroxyphenyl) -2 H -benzotriazoles (e.g. 2- (2 H -enzotriazol-2-yl) -4,6-bis (2-phenyl-2-propanyl) phenol and 2- (2 H -Benzotriazol-2-yl) -4,6-di- tert -butylphenol )
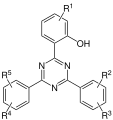
- (2-hydroxyphenyl) - s -triazines
- Hydroxybenzophenones
- Oxalanilides
Inorganic UV absorbers:
- Titanium dioxide
- Iron oxide pigments
- zinc oxide
- Compounds (for example stearates such as cadmium stearate ) based on lead , cadmium tin , barium / zinc , calcium / zinc and calcium / aluminum / zinc
criticism
Some commonly used substances, such as. B. benzophenone-4 , 3-benzylidenecamphor (3-BC), 4-methylbenzylidenecamphor , but also have a hormone-like ( estrogenic , i.e. feminizing) effect, which is why they are considered environmental chemicals and are counted among the endocrine disruptors . In fish (e.g. in males of Pimephales promelas ) from a concentration of 73 µg / L 3-BC causes the formation of the egg yolk protein vitellogenin (VTG). In ecotoxicology, VTG is a reliable biomarker for feminization.
Zinc oxide UV absorbers are based on nanoparticles of microfine zinc oxide . While such UV absorbers are not approved in Switzerland, the German Federal Institute for Risk Assessment (BfR) has so far had no health concerns if sunscreens contain a maximum of 25% microfine zinc oxide. However, the BfR notes that these particles can penetrate the skin in small quantities. Zinc, which comes from zinc oxide particles, has been found in small amounts in the blood and urine.
In the United States criticized that the population of access to approved in Europe UV absorbers such as amiloxate , Bemotrizinol , Iscotrizinol or octyl 'll denied so that the rising rates of skin cancer can be fought only inadequate.
Radical scavengers
Radical scavengers are not used to absorb UV radiation, but rather reduce the damage caused by UV radiation. This is i. d. Usually about radicals , which arise when chemical bonds are broken down by UV radiation. The largest group of radical scavengers are the so-called hindered amine light stabilizers (HALS).
Hindered Amine Light Stabilizers
Hindered Amine Light Stabilizers (HALS, roughly translated as: sterically hindered amines as light stabilizers ) are chemical compounds that contain amines as a functional group and that are used as UV stabilizers in polymers (plastics) and paints or coatings . These compounds are typically derivatives of tetramethylpiperidine and are mainly used to protect the polymers from the effects of photooxidation , as opposed to other forms of polymer degradation such as ozonolysis .
HALS are also increasingly used as thermal stabilizers, especially for low and moderate amounts of heat, but remain less effective than conventional phenolic antioxidants during high temperature processing of polymers (e.g. injection molding ) .
In addition to being used in the injection molding of polymers, HALS are also used in paints and other coating materials. Here they are used above all to give the paints sufficient weathering stability (especially in relation to solar radiation).
Mechanism of action of HALS
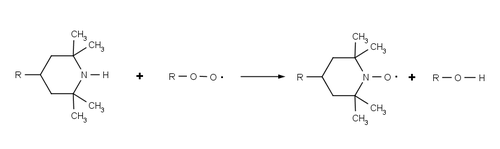
HALS do not absorb UV radiation , but rather inhibit the degradation of the polymer by continuously and cyclically removing radicals that are generated by photooxidation of the polymer. The overall process is sometimes referred to as the Denisov cycle after Yevgeny Timofejewitsch Denisov and is extremely complex. Often, HALS react with the starting polymer peroxy radicals (ROO •) and alkyl polymer radicals (R •) formed by the reaction of polymer and oxygen, which prevents further radical oxidation. These reactions oxidize HALS to their corresponding aminoxyl radicals (R 2 NO • cf TEMPO ), but they can revert to their original amine form via a series of additional radical reactions. The high efficiency and longevity of HALS are based on this cyclical process in which the HALS are regenerated during the stabilization process and are not used up.
Using a hindered amine that does not have alpha hydrogen atoms prevents the HALS from being converted to a nitrone . The formation of the nitrone may either dimerize or with any alkene groups in the polymer ( nitrones-olefin (3 + 2) cycloaddition ) happen is inactivated whereby the HALS.
Although HALS are extremely effective in polyolefins , polyethylenes, and polyurethanes , they are ineffective in polyvinyl chloride (PVC). It is believed that its ability to form nitroxyl radicals is impaired by the fact that it is easily protonated by HCl released by the dehydrohalogenation of PVC.
Examples of NECK
- 2,2,6,6-Tetramethylpiperidine derivatives such as bis (2,2,6,6-tetramethyl-4-piperidyl) sebacate (Tinuvin® 770)
Applications
When using light stabilizers, plastics (especially elastomers such as rubber ), textiles and paints need protection. A combination of UV absorbers and radical scavengers is often used. The use of UV absorbers is necessary to protect the layer or the underlying component or the human skin from UV radiation. However, since it is in a thin layer on the surface, e.g. B. a varnish, the UV radiation has not yet been sufficiently absorbed, the use of radical scavengers is also necessary here.
Plastics
Plastics are by the action of light photolysis a photooxidation subjected. This creates various radicals that attack the material. This breaks up the main chains of the polymer and incorporates various polar compounds such as peroxides . The consequences of this degradation are loss of color, cracking and generally negative effects on the physical mechanical properties.
Lacquers
UV absorbers and radical scavengers are used in paints to improve weathering stability . In clear coats in particular , it is important that the UV stabilizers used are colorless and not subject to any color changes. Due to the tendency to migrate , the basecoat in two-coat systems is also equipped with UV stabilizers, although it is already protected from ultraviolet radiation by a stabilized topcoat . For metallic coatings of automobiles, the durability of color, gloss and crack resistance can be guaranteed for periods of more than 10 years with the skillful use of HALS and UV absorbers.
Protection of human skin
The sunscreens used to protect human skin are mostly called sunscreens . They are designed to prevent the harmful effects of sun rays or artificial UV radiation on the skin.
See also
Web links
- UV-light stabilizers Hindered amine light stabilizers (english)
- flexorb Gas treating (english)
further reading
- Streitberger, Hans-Joachim; Goldschmidt, Artur: BASF Handbook Painting Technology. ISBN 978-3-86630-892-3 .
- Valet, Andreas: Sunscreens. ISBN 978-3-87870-437-9 .
- Müller, Bodo: Lacquer additives in a nutshell. ISBN 978-3-86630-695-0 .
- Johannes Karl Fink: A Concise Introduction to Additives for Thermoplastic Polymers . ISBN 0-470-60955-9 .
Individual evidence
- ^ U. Leiter, C. Garbe: Epidemiology of melanoma and nonmelanoma skin cancer - the role of sunlight. In: Advances in Experimental Medicine and Biology . Volume 624, 2008, pp. 89-103; doi : 10.1007 / 978-0-387-77574-6_8 . PMID 18348450 .
- ↑ GJ Nohynek, H. Schaefer: Benefit and Risk of organic ultraviolet filters. In: Regulatory Toxicology and Pharmacology . Volume 33, Number 3, June 2001, pp. 285-299; doi: 10.1006 / rtph.2001.1476 . PMID 11407932 .
- ↑ NA Quatrano, JG Dinulos: Current Principles of sunscreen use in children. In: Current Opinion in Pediatrics . Volume 25, Number 1, February 2013, pp. 122-129; doi: 10.1097 / MOP.0b013e32835c2b57 . PMID 23295720 .
- ↑ L. Scherschun, HW Lim: Photoprotection by sunscreens. In: American Journal of Clinical Dermatology . Volume 2, Number 3, 2001, pp. 131-134, ISSN 1175-0561 . PMID 11705089 .
- ^ Ultraviolet Absorbers ( Memento of May 2, 2009 in the Internet Archive ).
- ↑ T. Werner: Triplet deactivation in benzotriazole-type ultraviolet stabilizers . In: The Journal of Physical Chemistry . tape 83 , no. 3 , 1979, pp. 320-325 , doi : 10.1021 / j100466a004 .
- ↑ hessen-nanotech.de: Protection against radiation and temperature influences - UV protective layers , accessed on April 12, 2013.
- ↑ J. Winkler; Titanium dioxide ; Vincentz Network; Hanover; 2003; ISBN 3-87870-738-X
- ↑ G. Buxbaum, G. Pfaff; Industrial inorganic pigments; 3. Edition; Wiley-VCH; Weinheim; 2005; ISBN 3-527-30363-4
- ↑ Fabiola Moreno-Olivas, Elad Tako and Gretchen J. Mahler: ZnO nanoparticles affect intestinal function in an in vitro model . In: Food & Function . 2018, doi: 10.1039 / C7FO02038D .
- ↑ Entry on UV absorber. In: Römpp Online . Georg Thieme Verlag, accessed on January 25, 2020.
- ↑ General report on treatment and recovery routes for PVC waste ; Federal Ministry of Agriculture, Forestry, Environment and Water Management, Vienna, December, 2002, (PDF file)
- ↑ UV filters in cosmetics and in material protection. In: Karl Fent: Ecotoxicology. 4th, completely revised edition. Stuttgart 2013, ISBN 978-3-13-109994-5 , pp. 309f.
- ↑ Margret Schlumpf et al .: Estrogenic activity and estrogen receptor beta binding of the UV filter 3-benzylidene camphor. Comparison with 4-methylbenzylidene camphor. In: Toxicology . 199, 2004, pp. 109-120. PMID 15147785 .
- ↑ Tab. 9.9, Effects of estrogenic environmental substances on fish in vivo and PNEC values. In: Karl Fent: Ecotoxicology. 4th, completely revised edition. , Stuttgart 2013, ISBN 978-3-13-109994-5 , p. 306.
- ↑ Sunscreen: Zinc oxide as a UV filter is harmless to health according to the current state of knowledge. (PDF; 44 kB) BfR Opinion No. 037/2010 of June 18, 2010.
- ↑ B. Gulson, M. McCall, M. Korsch, L. Gomez, P. Casey, Y. Oytam, A. Taylor, M. McCulloch, J. Trotter, L. Kinsley, G. Greenoak: Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin. In: Toxicological Sciences . 118 (1), 2010, pp. 140-149. doi: 10.1093 / toxsci / kfq243 .
- ↑ Marc S. Reisch: After More Than A Decade, FDA Still Won't Allow New Sunscreens . In: Chemical & Engineering News . 93 (20), 2015, pp. 10–15.
- ↑ Hans Zweifel, Ralph D. Meier, Michael Schiller: Plastics additives handbook . 6th edition. Hanser, Munich 2009, ISBN 978-3-446-40801-2 .
- ↑ Klaus Köhler, Peter Simmendinger, Wolfgang Roelle, Wilfried Scholz, Andreas Valet, Mario Slongo: Paints and Coatings, 4. Pigments, Extenders, and Additives , in: Ullmann's Encyclopedia Of Industrial Chemistry . 2010, doi : 10.1002 / 14356007.o18_o03 .
- ↑ Pieter Gijsman: Photochemistry and Photobiology Physics of polymer material Photochemistry . John Wiley & Sons, Hoboken, doi : 10.1002 / 9780470594179.ch17 .
- ↑ Pieter Gijsman: A review on the mechanism of action and applicability of Hindered Amine Stabilizers . In: Polymer Degradation and Stability . 145, November 2017, pp. 2–10. doi : 10.1016 / j.polymdegradstab.2017.05.012 .
- ↑ R Gensler, CJG Plummer, H.-H Kausch, E Kramer, J.-R Pauquet, H Zweifel: Thermo-oxidative degradation of isotactic polypropylene at high temperatures: phenolic antioxidants versus HAS . In: Polymer Degradation and Stability . 67, No. 2, February 2000, pp. 195-208. doi : 10.1016 / S0141-3910 (99) 00113-5 .
- ↑ Streitberger, Hans-Joachim; Goldschmidt, Artur: BASF Handbook Painting Technology . Ed .: Vincentz. 2nd ed., Rev. Vincentz Network, Hannover 2014, ISBN 978-3-86630-892-3 , pp. 194 .
- ^ ET Denisov: The role and reactions of nitroxyl radicals in hindered piperidine light stabilization . In: Polymer Degradation and Stability . 34, No. 1-3, January 1991, pp. 325-332. doi : 10.1016 / 0141-3910 (91) 90126-C .
- ↑ Jennifer L. Hodgson, Michelle L. Coote: Clarifying the Mechanism of the Denisov Cycle: How do Hindered Amine Light Stabilizers Protect Polymer Coatings from Photo-oxidative Degradation? . In: Macromolecules . 43, No. 10, May 25, 2010, pp. 4573-4583. bibcode : 2010MaMol..43.4573H . doi : 10.1021 / ma100453d .
- ↑ Otto-Albrecht Neumüller (Ed.): Römpps Chemie-Lexikon. Volume 3: H-L. 8th revised and expanded edition. Franckh'sche Verlagshandlung, Stuttgart 1983, ISBN 3-440-04513-7 , pp. 2363-2364.
- ^ Ultraviolet Absorbers ( Memento of May 2, 2009 in the Internet Archive ).
- ^ A. Goldschmidt, H. Streitberger: BASF handbook paint technology . Vincentz Network, Hannover 2002, ISBN 3-87870-324-4 , p. 191 ff .
- ↑ Streitberger, Hans-Joachim; Goldschmidt, Artur: BASF Handbook Painting Technology . Ed .: Vincentz. 2nd ed., Rev. Vincentz Network, Hannover 2014, ISBN 978-3-86630-892-3 , pp. 194 .
- ^ Brockhaus ABC Chemie , VEB FA Brockhaus Verlag Leipzig 1965, p. 1310.