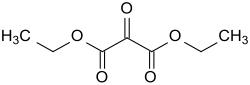
Diethyl mesoxalate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Diethyl mesoxalate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 7 H 10 O 5 | |||||||||||||||
| Brief description |
clear colorless to yellow liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 174.15 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density | ||||||||||||||||
| Melting point |
−30 ° C |
|||||||||||||||
| boiling point |
|
|||||||||||||||
| solubility |
very soluble in water, soluble in ethanol , diethyl ether , chloroform |
|||||||||||||||
| Refractive index |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Mesoxalsäurediethylester is the diethyl ester of mesoxalic acid (ketomalonic) the simplest Oxodicarbonsäure and thus the first member (n = 0) of a homologous series HOOC-CO- (CH 2 ) n -COOH with the higher homologs of oxaloacetic acid (n = 1) α- Ketoglutaric acid (n = 2) and α-ketoadipic acid (n = 3), a metabolite of the amino acid lysine .
Because of its strongly polarized keto group, diethyl mesoxalate reacts as an electrophile in addition reactions and is a highly active reactant in pericyclic reactions , e.g. B. in Diels-Alder reactions , cycloadditions or ene reactions .
In moist air, mesoxalic acid diethyl ester water accumulates to form the so-called diethyl mesoxalate hydrate (2,2-dihydroxymalonic acid diethyl ester) and the green-yellow oil changes spontaneously into white crystals.
Occurrence and representation
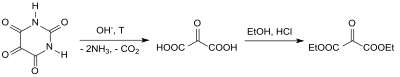
In 1892 Richard Anschütz and co-workers presented the so-called oxomalonic acid ethyl ester for the first time, based on the barium salt of the alloxan , its decomposition to mesoxalic acid and esterification with ethanol in the presence of hydrogen chloride , in pure form.
Louis Bouveault and co-workers obtained isonitrosoester via nitrosation of diethyl malonate with nitrosylsulfuric acid , which is oxidized to diethyl mesoxalate with dinitrogen tetroxide N 2 O 4 (“peroxyde d'azote”). The keto compound obtained as an oil reacts with water to form the crystalline dihydrate.
In a modified variant of the synthesis with N 2 O 4 , diethyl mesoxalate was obtained in a crude yield of 90%.
Instead of dinitrogen tetroxide, dinitrogen trioxide N 2 O 3 ( obtainable from arsenic (III) oxide with nitric acid ) can also be used as the oxidizing agent. The yield in the final stage is 74-76%; however, the synthetic route is expensive in terms of equipment and unsuitable because of the toxicity and carcinogenicity of As 2 O 3 .
The oxidation of malonic ester with selenium dioxide SeO 2 is unproductive with a yield of ester hydrate of 23%, as is the reaction indicated as "improved synthesis" via the malonic ester dibromide and elimination of bromide with potassium acetate with a yield of 41 to 47%.
Several processes for the production of ethyl mesoxalate make use of the oxidation of diethyl malonate or its enamines with oxygen or ozone . The ozonolysis of diethylethylidene malonate (from malonic ester and acetaldehyde in approx. 80% yield) at −78 ° C provides only 62% diethyl mesoxalate, while the electrochemical oxidation of diethyl cyanmalonate (from ethyl cyanoacetate and ethyl chloroacetate with the aid of oxygen 77% yield) in the last oxidation stage and the ozonolysis of dialkyl benzal malonates given by Lutz Friedjan Tietze using the example of the dimethyl ester, 76% of the dimethyl mesoxalate.
Because of the risks involved in handling ozone, ozonolysis is essentially limited to the laboratory scale (up to about 150 g of product).
The photooxidation of the enamine formed from dimethylformamide dimethylacetal and malonic acid diethyl ester in practically quantitative yield produces diethyloxomalonate hydrate in 84% yield.
A more recent patent describes the synthesis of diethyl mesoxalate from the simple diethyl malonate precursor by oxidation with aqueous sodium chlorite (NaClO 2 ) solution at pH 4.4 in 97% yield.
The ester is initially obtained as a hydrate, which is dehydrated to the end product by azeotropic distillation with toluene .
properties
Diethyl mesoxalate is a greenish-yellow, low-viscosity oil with a faint odor that quickly crystallizes with water to form a solid dihydrate in coarse white prisms.
Applications
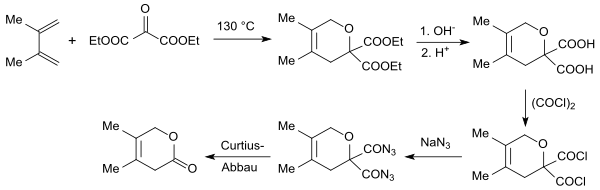
The electron-poor dienophile diethyl ketomalonate is suitable as a carbon dioxide equivalent for Diels-Alder reactions with electron-rich 1,3-dienes , such as. B. with isoprene or 2,3-dimethylbutadiene in a [4 + 2] - cycloaddition to geminal dihydropyran diester, which hydrolyzes alkaline to gem -diacid, halogenated with oxalyl chloride to gem -diacid dichloride, with sodium azide into gem -diacid diazide and converts this into a Curtius reaction can be broken down to a dihydropyranone.
In an aldol diethyl mesoxalate reacts with the Morpholinenamin of 3-pentanone to an α-hydroxy-γ-ketodiester, which with phosphorus pentoxide / methanesulfonic acid , a mixture substituted butenolide forms.
With guanidine , a functionalized imidazolone is formed in 85% yield.
Diethyl ketomalonate is a versatile reactant in the Baylis-Hillman reaction and forms the corresponding multifunctional compounds with acrylic acid esters , acrylonitrile or methyl vinyl ketone under catalysis with DABCO .
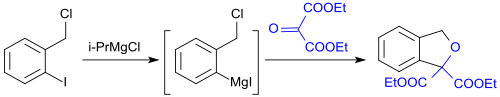
Diethyl mesoxalate reacts with the Grignard compound formed from 1-iodo-2-chloromethylbenzene and isopropyl magnesium chloride to form 2-bis-carboxyethyl-isobenzofuran.
Diethyl mesoxalate adds in an ene reaction to terminal double bonds of alkenes to form 1-hydroxy-1-alkylmalonic esters.
Individual evidence
- ↑ a b c T.F. Tietze, C. Schneider, DJ Coughlin: Diethyl Oxomalonate . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2009, doi : 10.1002 / 047084289X.rd207m.pub2 .
- ↑ a b c d e data sheet Diethyl ketomalonate from Sigma-Aldrich , accessed on November 15, 2017 ( PDF ).
- ↑ a b c W.M. Haynes: CRC Handbook of Chemistry and Physics, 96th Edition . CRC Press, Boca Raton, FL, USA 2015, ISBN 978-1-4822-6097-7 , pp. 3-178 .
- ↑ Data sheet Diethyl ketomalonate from AlfaAesar, accessed on November 15, 2017 ( PDF )(JavaScript required) .
- ↑ a b R. Müller: On the knowledge of the specific oxidizing effect of selenium dioxide . In: Ber. German Chem. Ges. Volume 66 , no. 11 , 1933, pp. 1668-1670 , doi : 10.1002 / cber.19330661111 .
- ↑ a b R. Anschütz, E. Parlato: Ueber den Oxomalonsäureäthylester . In: Ber. German Chem. Ges. Volume 25 , no. 2 , 1892, p. 3614-3617 , doi : 10.1002 / cber.189202502245 .
- ↑ L. Bouveault, A. Wahl: Sur les éthers isonitrosomalonique et leur transformation en éthers mésoxaliques . In: CR Acad. Sci. tape 138 , 1903, pp. 196-198 .
- ↑ E. Gilman, TB Johnson: The synthesis of mesoxalates by interaction of nitrogen tetroxide with esters of malonic acid . In: J. Am. Chem. Soc. tape 50 , no. 12 , 1928, pp. 3341–3348 , doi : 10.1021 / ja01399a028 .
- ↑ AW Dox: Ethyl oxomalonate In: Organic Syntheses . 4, 1925, p. 27, doi : 10.15227 / orgsyn.004.0027 ; Coll. Vol. 1, 1941, p. 266 ( PDF ).
- ↑ SN Pardo, RG Salomon: Diethyl malonate. An improved synthesis . In: J. Org. Chem. Band 46 , no. 12 , 1981, p. 2598-2599 , doi : 10.1021 / ja00325a039 .
- ^ ME Jung, K. Shishido, LH Davis: Simple syntheses of diethyl oxomalonate and alkyl glyoxylate . In: J. Org. Chem. Band 47 , no. 5 , 1982, pp. 891-892 , doi : 10.1021 / jo00344a028 .
- ↑ M. Sugawara, MM Baizer: Electrogenerated bases VII. Novel syntheses of ethyl glyoxalate and diethyl ketomalonate via electrogenerated superoxide . In: Tetrahedron Lett. tape 24 , no. 22 , 1983, pp. 2223-2266 , doi : 10.1016 / S0040-4039 (00) 81889-4 .
- ↑ LF Tietze, M. Bratz: Dialkyl mesoxalates by ozonolysis of dialkyl benzalmalonates: Dimethyl mesoxalate In: Organic Syntheses . 71, 1993, p. 214, doi : 10.15227 / orgsyn.071.0214 ; Coll. Vol. 9, 1998, p. 314 ( PDF ).
- ↑ HH Wasserman, WT Han: Vicinal tricarbonyl products from singlet oxygen reactions .: Application to the synthesis of carbacephams . In: Tetrahedron Lett. tape 25 , no. 34 , 1984, pp. 3743-3746 , doi : 10.1016 / 0040-4039 (84) 80120-3 .
- ↑ Patent US8859803B2 : Process for production of ketomalonic acid compounds or hydrates thereof. Filed June 25, 2010 , published October 14, 2014 , applicant: Ihara Chemical Industry Co., Ltd., inventor: S. Tani.
- ↑ RA Ruden, R. Bonjouklian: Carbon dioxide equivalent for the Diels-Alder reaction . In: J. Am. Chem. Soc. tape 97 , no. 23 , 1975, p. 6892-6893 , doi : 10.1021 / ja00856a063 .
- ↑ R. Bonjouklian, RA Ruden: Versatile allene and carbon dioxide equivalents for the Diels-Alder reaction . In: J. Org. Chem. Band 42 , no. 25 , 1977, pp. 4095-4103 , doi : 10.1021 / jo00445a024 .
- ↑ AG Schultz, YK Yee: Synthesis of α-carbalkoxy-γ-alkylidene-Δ α , β- butenolide . In: J. Org. Chem. Band 41 , no. 3 , 1976, p. 561-563 , doi : 10.1021 / jo00865a035 .
- ↑ C. Quirosa-Guillou, DZ Renko, C. Thal: Réaction des guanidines avec les composés tricarbonylés vicinaux: new accès aux composés à squelette 2-aminoimidazolique . In: Tetrahedron . tape 48 , no. 31 , 1992, pp. 6385-6392 , doi : 10.1016 / S0040-4020 (01) 88228-4 .
- ↑ D. Basavaiah, VVL Gownswari: Diethyl ketomalonate: A fast reacting substrate for Baylis-Hillman reaction . In: Synth. Commun. tape 19 , no. 13-14 , 1989, pp. 2461-2465 , doi : 10.1080 / 00397918908052648 .
- ↑ BN Rocke, EL Conn, SA Eisenbeis, RB Ruggeri: 1,4-Addition of an aryllithium reagent to diethyl ketomalonate. Scalable synthesis of ethyl 1- (hydroxymethyl) -1,3-dihydroisobenzofuran-1-carboxylate . In: Tetrahedron Lett. tape 53 , no. 41 , 2012, p. 5467–5470 , doi : 10.1016 / j.tetlet.2012.05.052 .
- ↑ Patent US6730747B1 : carbonyl compounds Containing. Filed January 4, 2000 , published May 4, 2004 , applicant: Infineum USA LP, inventor: SJ Brois.