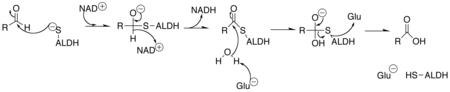Aldehyde dehydrogenase 2
| Aldehyde dehydrogenase 2 | ||
|---|---|---|

|
||
| Octamer according to PDB 1CW3 | ||
|
Existing structural data : 1a4z , 1ag8 , 1cw3 , 1nzw , 1nzx , 1nzz , 1o00 , 1o01 , 1o02 , 1o04 , 1o05 , 1of7 , 1zum , 2onm , 2onn , 2ono , 2onp |
||
| Properties of human protein | ||
| Mass / length primary structure | 500 amino acids; 54.4 kDa | |
| Secondary to quaternary structure | Homotetramer | |
| Isoforms | ALDH1 | |
| Identifier | ||
| Gene names | ALDH2 ; ALDH-E2; ALDHI; ALDM; MGC1806 | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 1.2.1.3 , oxidoreductases | |
| Substrate | Aldehyde + NAD + + H 2 O | |
| Products | Carboxylic acid + NADH | |
| Occurrence | ||
| Homology family | HBG439831 | |
| Parent taxon | Creature | |
| Orthologue | ||
| human | mouse | |
| Entrez | 217 | 11669 |
| Ensemble | ENSG00000111275 | ENSMUSG00000029455 |
| UniProt | P05091 | Q3TVM2 |
| Refseq (mRNA) | NM_000690 | NM_009656 |
| Refseq (protein) | NP_000681 | NP_033786 |
| Gene locus | Chr 12: 110.69 - 110.73 Mb | Chr 5: 121.83 - 121.85 Mb |
| PubMed search | 217 |
11669
|
Aldehyde dehydrogenase 2 (ALDH-2) is an enzyme belonging to the group of aldehyde dehydrogenases , which is required in the human body to break down alcohol ( ethanol ). ALDH-2 converts the toxic acetaldehyde (ethanal) - produced by ADH from alcohol - into acetate (ethanoic acid).
properties
Aldehyde dehydrogenase is mainly known as an alcohol-degrading enzyme . Its mitochondrial isoform , mtALDH2, primarily has a protective effect on the heart muscle . Its cardioprotective activity is stopped after an oxygen deficiency situation in the heart, e.g. B. by an occlusion of the coronary vessels , for the survival of the heart muscle cells of great importance. The ADH2 protects the tissue and the cells from the damaging effects of alcohol or acetaldehyde , and other toxic aldehydes . mtALDH2 plays an important role in the detoxification of reactive oxygen species ( Engl . R eactive O xygen S pecies , ROS), and is thereby instrumental in maintaining an intact function of the mitochondria involved. It is therefore very common in the heart, an organ that contains a particularly large number of mitochondria and is therefore sensitive to oxidative stress and ROS. The activation of mtALDH2 mainly prevents premature apoptosis and necrosis of the heart muscle cells as well as the formation of fibrotic tissue.
46 percent of the Japanese and 56 percent of the Chinese are affected by a polymorphism of acetaldehyde dehydrogenase 2. They are carriers of a dominant allele of the ALDH2 gene, in which the glutamate is exchanged for lysine at position 487 of the amino acid sequence . The modified ALDH2 can process acetaldehyde less effectively and is itself broken down more quickly. This makes it easier for acetaldehyde to accumulate in the body and thus for the symptoms of intoxication associated with excessive alcohol consumption ( flush syndrome ). The affected people are therefore more sensitive to the negative effects of alcohol consumption.
Some lactic acid bacteria also chart the opposite way with the aldehyde dehydrogenase 2: Under good conditions, they build the whole of the glycolysis -derived pyruvate to lactate from. However, if there is a lack of glucose , various homofermentative strains split the pyruvate into formate and acetyl-coenzyme A using pyruvate formate lyase . Half of the acetyl-CoA can now be converted by the aldehyde dehydrogenase into acetaldehyde, which the alcohol dehydrogenase converts into ethanol. The other half of the acetyl-CoA is used to produce acetate, which can be used for ATP synthesis.
The role of ROS and the activation of mtALDH2
mtALDH2 is a key enzyme in ischemic preconditioning . If the myocardium is preconditioned by short ischemic phases that are harmless to the heart and this is followed by a severe infarction , the damage to the myocardium caused by the lack of oxygen is significantly less than after an infarct without preconditioning.
The result of the preconditioning is the activation of mtALDH2. Preconditioning can also be caused by chemical substances such as ethanol or anesthetics . An important activator of mtALDH2 in preconditioning is protein kinase - ε (PKC-ε). This is used by the RISK (engl .: R epefusions- I njury- S ignalling- K inases ) by phosphorylation activated. RISK itself are activated by various preconditioning mechanisms. The PKC-ε activated by RISK migrates from the cytosol into the mitochondrion in order to activate mtALDH2 there by phosphorylation.
Formation of ROS and lipid peroxidation
A particularly important task of mtALDH2 is to remove the reactive aldehydes that are harmful to the cell. It is in the detoxification of reactive aldehydes such. B. 4-hydroxynonenal (4-HNE) or malondialdehyde (MDA) involved.
Reactive aldehydes arise in the myocardium mainly during the reperfusion phase , when the tissue is suddenly supplied with blood and oxygen again after ischemia has resolved. This sudden return of oxygen leads to oxidative stress, a condition in which more ROS is formed in the cell than is broken down. ROS oxidize unsaturated fatty acids such as B. arachidonic acid and linoleic acid with peroxides whereby u. a. the mitochondrial membrane is damaged and reactive aldehydes such as 4-HNE are formed. This process is known as lipid peroxidation . The aldehydes formed from lipid peroxidation are by their unsaturated α / β - carbon atoms very reactive and can therefore by reaction with the amino acid residues of cysteine , histidine or lysine of proteins , cell-damaging protein adducts are formed.
Reactive aldehydes inhibit the electron transport chain in the mitochondrion and induce the opening of the mitochondrial permeability transition pore (mPTP). The attack on the mitochondrial membrane also causes the mitochondria to dysfunction, causing them to produce even more ROS. In addition, if the mitochondrial membrane is not intact, the enzymes located on the inner mitochondrial membrane, especially cytochrome C , are released. The release of cytochrome C leads to the formation of an apoptosome and thus to cell death. mtALDH2 interrupts the spiral of ROS formation and mitochondrial damage by inhibiting the formation of ROS and the blocking of the electron transport chain at complex I and IV as well as the Ca 2+ -induced opening of the mPTP.
Ischemia reperfusion damage - IR damage : The occurrence in which the cell membrane and subsequently the entire tissue is damaged, both by the lack of oxygen during ischemia and by the subsequent reperfusion of the tissue, is the cause of ischemia reperfusion - ( IR ) damage .
ROS mediated activation of PKC-δ
As already stated in the introduction, the PKC-ε has a positive effect on an IR event by activating the mtALDH2. Its isoform , the protein kinase- δ (PKC-δ), however, participates in the opposite process. PKC-δ is activated by ROS and then translocates from the cytosol into the mitochondrion.
There, PKC-δ mediates the activating phosphorylation on serine 616 of the Dynamin-related protein 1 (Drp1) via a cAMP- dependent protein kinase . Drp1 is responsible for the natural splitting (fission) of two mitochondria that have fused as part of their recycling. The increased activity of Drp1, however, leads to an excessive division into two parts and thus to the removal of individually existing mitochondria that are destined to breakdown. Besides the phosphorylation of Drp1, the activation of PKC-δ leads to the activation of glycogen synthase kinase-3β (GSK-3β). If the GSK-3β is switched on, it causes u. a. the opening of the mPTP and thus the induction of apoptosis.
mtALDH2 is also gaining importance in the process of inhibiting PKC-δ because it is essential for the detoxification of ROS. It eliminates ROS, thereby removing an activator from PKC-δ. Another aspect of the inhibition is the induction of PKC-ε. Their activation by the RISK leads to a negative feedback on the expression and translocation of their isoenzyme PKC-δ.
Cardioprotective effects of ethanol
mtALDH2 acts as an enzyme both in alcohol metabolism and in the breakdown of reactive aldehydes. Based on this fact it seems possible that an alcohol-induced activation of mtALDH2 has a positive effect on the protection of the heart muscle.
In fact, after moderate alcohol consumption, an increased activity of mtALDH2 can be determined. This preconditioning with alcohol leads to a high concentration of already activated mtALDH2 in an IR event. An increased level of active mtALDH2 expands the cell's capacity to break down reactive aldehydes and to inhibit lipid peroxidation. Another cardioprotective effect of alcohol is the activation of superoxide dismutase (SOD). SOD is an important myocardial antioxidant that detoxifies and removes dangerous ROS from heart muscle cells. In Asia, 40% of the population are carriers of a mutation of the ALDH2 allele . They have the ALDH2 * 2 mutant, which is less active than the wild type . Carriers of ALDH2 * 2 are exposed to a higher risk of suffering cardiac damage from chronic alcohol consumption.
Inhibition of apoptosis
Although the rapid reperfusion of the heart muscle can significantly reduce the spread of the infarct area through a rapid re-supply of oxygen, paradoxically, the return of oxygen itself also causes damage to the myocardium (IR damage).
During the ischemic phase, the cells of the heart muscle perish due to necrosis. However, during reperfusion the cells die by apoptosis. This is related to the fact that apoptosis is an ATP dependent process. During ischemia, the production of ATP is markedly reduced by blocking the oxygen-dependent ATP synthesis pathways from hypoxia . The lack of ATP largely prevents cell death through apoptosis. The reperfusion supplies the tissue with oxygen and glucose again . The return of oxygen ensures, on the one hand, that the ATP concentration rises again; on the other hand, the high oxygen supply leads to oxidative stress. The combination of ROS and ATP formation promotes cell death through apoptosis. At this point mtALDH2 has a cardioprotective effect by inhibiting the formation of ROS and thus counteracting apoptosis. In addition, mtALDH2 inhibits a signaling pathway of apoptosis by increasing the activity of Akt and of AMP-dependent protein kinase (AMPK), which then inhibit their apoptotic target enzymes Foxo3 and Caspase-3 .
Autophagy
In addition to necrosis and apoptosis, autophagy also contributes to the fact that the cardiac muscle tissue is restricted in its function after ischemia with subsequent reperfusion. Autophagy is a natural mechanism of the cells in which the survival and death of the cell is regulated by the breakdown of cellular material. However, this process can be increased excessively by 4-HNE. 4-HNE accumulates in the heart muscle cells due to the influence of ROS. It regulates the autophagy of the cardiac muscle cells during ischemia and reperfusion in the opposite direction.
Normally, during ischemia, there is more autophagy to protect the myocardium . Here the inhibitor of autophagy, mTOR , is inhibited by LKB1 ( liver kinase B1) or AMPK, so that autophagy is possible. During reperfusion, however, mTOR is activated by PTEN or Akt, so that mTOR now inhibits autopgagie. This will prevent excessive autophagy during reperfusion. However, 4-HNE blocks both PTEN and LKB1. As a result, mTOR is active during ischemia on the one hand, so that autophagy, which is beneficial during the oxygen deficiency phase, is inhibited. On the other hand, it is not activated during reperfusion and therefore cannot inhibit autophagy at this point. mtALDH counteracts the dysregulation of 4-HNE by curbing the formation of 4-HNE and promoting its breakdown.
Mitophagy
mtALDH2 also prevents mitophagy, which is inhibited by oxidative stress, i.e. the breakdown of damaged mitochondria. Damaged mitochondria have to be broken down to protect the cell. Oxidative stress leads to the inactivation of parkin , which is important for the breakdown of the mitochondrion, and thus prevents the removal of the damaged mitochondrion from the cell. If the malfunctioning mitochondria are not broken down, they form more ROS, which subsequently leads to oxidative stress. mtALDH2 protects the cell from inactivation of mitophagy by inhibiting the development of ROS and the associated inactivation of parkin.
ER stress
Due to the hypoxic conditions during ischemia, the protein balance of the myocardial cells, in particular protein folding and protein breakdown , is severely disturbed. Various stressors, such as B. Hypoxia causes the endoplasmic reticulum (ER) to decrease its capacity to correctly fold newly synthesized proteins and to correctly fold incorrectly folded proteins. Protein that needs to be folded therefore accumulates in the cell, and a condition called ER stress occurs . ER stress triggers apoptosis of the cardiomyocytes in the myocardium and thus reduces the heart muscle tissue by a large part of its functional cells.
ER stress leads to apoptosis via NADPH oxidase . So that the cell can survive despite ER stress, this apoptotic signaling pathway is inhibited by the Akt and PI3K signaling pathways that are coupled to a growth factor . Here Akt is phosphorylated by PI3K. The now activated Akt inhibits the p 47phox subunit of NADPH oxidase. If the p 47phox subunit were not inhibited, it would initiate apoptosis. This happens when the PI3K is inhibited by ER stress and thus the activation of Akt is prevented. Thus the p 47phox subunit remains active and initiates apoptosis. mtALDH2 maintains the signaling pathway of PI3K and Akt even during ER stress. It removes the inhibitory effect of ER stress on PI3K, so that Akt continues to be activated and inhibits apoptosis.
The chaperones and regulators that typically arise during ER stress, such as B. GRP78 and CHOP themselves also mediate apoptosis in a still unknown way. The apoptosis-inhibiting effect of mtALDH2 is to inhibit CHOP and GRP78. Another stressor causing ER stress is 4 HNE. mtALDH2 eliminates this stress factor by eliminating 4-HNE through detoxification.
Fibrosis
The formation of a fibrosis in the heart is the main cause of decreased cardiac output after a heart attack. She walks with a reduced contractility of the heart muscle and a reduced ejection fraction of the left ventricle ( L eft V entricular E jection F raction - LVEF ) accompanied. One reason for the change in myocardial tissue is an overactive Wnt / β-catenin signaling pathway. This signal pathway is responsible for the regeneration and repair of the tissue and is inactive or strongly regulated under physiological conditions. An infarction leads to its prolonged and excessive activation, as a result of which the myocardium and epicardium are transformed into highly fibrous, largely functionless tissue. Cardiac fibrosis develops on the one hand through the Wnt-mediated epithelial-mesenchymal transition (EMT) of the cells of the epicardium in fibroblasts . On the other hand, the myocardium is restructured by the infiltration of its infarct-damaged necrotic areas with fibroblasts into a less elastic and less contractile tissue.
Wnt / β-catenin signaling pathway
In the absence of Wnt is the transcription - coactivator β-catenin in a complex in which he Axin and APC - protein ( A denomatöses P olyposis C oli) with which CK1 ( Caseinkinase1 is linked), and GSK-3β. Bound in this complex, β-catenin is phosphorylated by CK1 and GSK-3β. The phosphorylated β-catenin is of the β-TrCP subunit of E3 ubiquitin - ligase recognized and ubiquitinated. Ubiquitination causes it to be broken down in the proteasome and thus continuously eliminated from the cytosol.
Wnt is present, then it formed a receptor complex with the 7- transmembrane receptor Frizzled , and the co-receptor LRP5 ( L ow-density lipoprotein (LDL) - R preceptor-Related- P rotein ) or LRP6. The protein Disheveled (Dsh), GSK-3β and CK1 now bind on the cytosolic side of the receptor complex. The two kinases GSK-3β and CK1 phosphorylate LRP so that Axin can be bound to LRP. Due to the binding of axin to the receptor complex in the membrane, β-catenin remains unphosphorylated in the cytosol and is not broken down. It migrates into the cell nucleus where, together with the T-cell factor (TCF), it switches on the transcription of its target genes. A major target gene is the W nt I nducible- S ignaling P athway (WISP) -1 gene. WISP-1 is a growth factor that stimulates the synthesis and release of collagen and the proliferation of fibroblasts.
In the presence of mtALDH2, the development of fibrosis is significantly reduced. In the absence of mtALDH2, the concentration of β-catenin, Wnt and active GSK-3β is increased. mtALDH2 inhibits the dephosphorylation of GSK-3β and thus prevents its activation, because phosphorylated GSK-3β is inactive. The process of stabilization of β-catenin is inhibited, whereby the signal cascade via the Wnt switches on its target genes is interrupted. The activation of mtALDH2 also leads to less collagen I and III being formed and accumulated in the cells. In addition, the expression of α-SMA (engl .: will S mooth M uscle A CTIN ) and WISP-1 inhibited. The function of mtALDH2 as an alcohol-degrading enzyme should also be remembered, because the acetylaldehyde that is produced during alcohol degradation and is degraded by mtALDH2 is known to promote fibrogenesis.
Activation of drugs by ALDH2
As a nitrate reductase, aldehyde dehydrogenase 2 is involved in the activation of two NO donors . Specifically, it catalyzes the release of nitric oxide from glycerol trinitrate and pentaerythrityl tetranitrate in the mitochondria of smooth muscle cells. The NO causes relaxation there, which causes the vessels to widen.
Individual evidence
- ↑ a b c d e f g h i j Pang Jiao-Jiao, Chen You-Go, Ren Jun: Mitochondrial aldehyde dehydrogenase in myocardial ischemia-reperfusion injury: from bench to bedside. In: Acta Physiologica Sinica ,. NCBI National Center for Biotechnology Information, December 25, 2015, pp. 335–344 , accessed April 1, 2017 .
- ↑ a b c d e f g J. Liao, A. Sun, Y. Xie, T. Isse, T. Kawamoto, Y. Zou, J. Ge: Aldehyde dehydrogenase-2 deficiency aggravates cardiac dysfunction elicited by endoplasmic reticulum stress induction . In: Molecular medicine. Volume 18, July 2012, pp. 785-793, doi: 10.2119 / molmed.2011.00466 , PMID 22430940 , PMC 3409283 (free full text).
- ↑ a b c d e f g h C. H. Chen, L. Sun, D. Mochly-Rosen: Mitochondrial aldehyde dehydrogenase and cardiac diseases. In: Cardiovascular research. Volume 88, number 1, October 2010, pp. 51-57, doi: 10.1093 / cvr / cvq192 , PMID 20558439 , PMC 2936126 (free full text) (review).
- ↑ Q. Xiao, H. Weiner, DW Crabb: The mutation in the mitochondrial aldehyde dehydrogenase (ALDH2) gene responsible for alcohol-induced flushing increases turnover of the enzyme tetramers in a dominant fashion. In: The Journal of clinical investigation. Volume 98, Number 9, November 1996, pp. 2027-2032, ISSN 0021-9738 . doi: 10.1172 / JCI119007 . PMID 8903321 . PMC 507646 (free full text).
- ^ A b C. E. Murry, RB Jennings, KA Reimer: Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium . In: Circulation . tape 74 , no. 5 , November 1, 1986, ISSN 0009-7322 , pp. 1124-1136 , PMID 3769170 .
- ↑ Xiao-E. Lang, Xiong Wang, Ke-Rang Zhang, Ji-Yuan Lv, Jian-Hua Jin: Isoflurane Preconditioning Confers Cardioprotection by Activation of ALDH2 . In: PLOS ONE . tape 8 , no. 2 , February 28, 2013, ISSN 1932-6203 , p. e52469 , doi : 10.1371 / journal.pone.0052469 , PMID 23468836 , PMC 3585331 (free full text).
- ↑ a b Shijun Wang, Feng Zhang, Gang Zhao, Yong Cheng, Ting Wu: Mitochondrial PKC-ε deficiency promotes I / R-mediated myocardial injury via GSK3β-dependent mitochondrial permeability transition pore opening . In: Journal of Cellular and Molecular Medicine . March 1, 2017, ISSN 1582-4934 , doi : 10.1111 / jcmm.13121 .
- ↑ Qing Yuan, Shanjuan Hong, Shu Han, Li Zeng, Fang Liu: Preconditioning with Physiological Levels of Ethanol Protect Kidney against Ischemia / Reperfusion Injury by Modulating Oxidative Stress . In: PLOS ONE . tape 6 , no. 10 , October 12, 2011, ISSN 1932-6203 , p. e25811 , doi : 10.1371 / journal.pone.0025811 , PMID 22022451 , PMC 3192120 (free full text).
- ↑ a b c d Xinjun Zhao, Yue Hua, Hongmei Chen, Haiyu Yang, Tao Zhang: Aldehyde dehydrogenase-2 protects against myocardial infarction-related cardiac fibrosis through modulation of the Wnt / β-catenin signaling pathway . In: Therapeutics and Clinical Risk Management . tape 11 , September 11, 2015, ISSN 1176-6336 , p. 1371-1381 , doi : 10.2147 / TCRM.S88297 , PMID 26392772 , PMC 4574798 (free full text).
- ^ A b Bryan T. MacDonald, Keiko Tamai, Xi He: Wnt / β-catenin signaling: components, mechanisms, and diseases . In: Developmental cell . tape 17 , no. 1 , April 6, 2017, ISSN 1534-5807 , p. 9–26 , doi : 10.1016 / j.devcel.2009.06.016 , PMID 19619488 , PMC 2861485 (free full text).
- ↑ Schubert-Zsilavecz, Manfred., Roth, Hermann J .: Medicinal Chemistry: Targets - Drugs - Chemical Biology; 191 tables . 2., completely reworked. and exp. Ed. Dt. Apotheker-Verl, Stuttgart 2010, ISBN 978-3-7692-5002-2 .