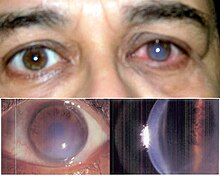
Keratoconus
| Classification according to ICD-10 | |
|---|---|
| H18.6 | Keratoconus |
| H19.8 | Keratoconus in Down syndrome |
| Q13.4 | Congenital keratoconus |
| Q13.9 | Congenital malformation of the anterior segment of the eye |
| Z94.7 | Condition after keratoplasty |
| ICD-10 online (WHO version 2019) | |
The eye disease keratoconus (from Greek κέρας keras 'horn', Latin conus 'cone') describes the progressive thinning and conical deformation of the cornea of the eye . The disease is always bilateral, but can be weaker in one eye or not become symptomatic at all (Forme Fruste Keratoconus). The disease is therefore characterized by two properties:
- Progression (development): The cornea becomes thinner and more pointed
- Visual impairment: Due to the irregular deformation of the cornea, the visual acuity decreases.
Usually those affected are nearsighted . However, this nearsightedness cannot be completely corrected with a visual aid , since the conical corneal protrusion causes an irregular corneal curvature ( astigmatism ).
history
The German ophthalmologist Burkhard David Mauchart described an illness in his doctoral thesis in 1748 , which he called "Staphylom diaphanum". However, it was not until 1854 that the British doctor John Nottingham described keratoconus in more detail and also found that it is clearly different from other ectasias of the cornea. Nottingham reported cases of " conical cornea" and described several features of the disease such as diplopia , corneal weakening, and difficulty seeing with corrective lenses.
In 1859, surgeon William Bowman used an ophthalmoscope ( invented shortly before by Hermann von Helmholtz ) to more accurately diagnose keratoconus. Bowman inserted a fine hook through the cornea, pulling on the iris and expanding the pupil into a vertical slit like a cat's. He was able to use this method successfully on an 18-year-old woman.
In 1869 the disorder got its current name from the Swiss ophthalmologist Johann Friedrich Horner .
Epidemiology
Keratoconus occurs in the countries of the western world in approx. 1 person per approx. 1000–2000 inhabitants. The frequency of occurrence is higher in countries of the Middle East and Asia. In Germany about 0.5 ‰ of the population, i. H. every 2000th (a total of around 40,000 people) affected, although this may vary depending on the region and examination methods. In Down syndrome , the number of cases is up to 15%.
The keratoconus usually becomes manifest between the ages of 20 and 30. But it can also become symptomatic from childhood up to the age of 40 or 50.
Symptoms
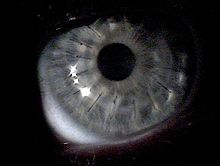
The first signs are frequent eyeglass corrections with a change in the axis and changing eyesight, as well as increasing corneal curvature, often initially on one eye. Because it is a rare disease, symptoms in the early stages are often not associated with this eye disease. Most of those affected have switched from different ophthalmologists for years until they come across one who recognizes the disease. With keratoconus, those affected see phenomena such as, in some cases only in one eye. For example: "Double vision of objects" ( monocular diplopia ) , additional shadows on letters and objects, as well as streaks or star-shaped rays that seem to emanate from light sources.
In the initial stage, a reliable assessment of the symptoms is only possible through an analysis of the corneal surface, the back surface of the cornea, the corneal thickness and, if necessary, a cell count.
Further characteristics are hemosiderin rings (Fleischer's rings), known as keratoconus lines. A yellow-brown to green-brown color appears here, which surrounds the base of the cone as a half or closed circle, visible in good lighting. During the further course of the disease, superficial, irregular scars and opacities as well as tears in Descemet's membrane can become visible and Vogt's lines appear.
If the disease is advanced, keratoconus can be seen from the side with the naked eye. If corneal edema (fluid build-up in the cornea) occurs, it is acute keratoconus . This can heal after three to four months with scarring.
Rarely, tears in the posterior cornea can occur, so that fluid from the anterior chamber penetrates the cornea, resulting in hydrops or acute keratoconus. This also manifests itself in an acute, severe clouding of the cornea (you can see fog ). The hydrops usually regresses by itself.
root cause
The cause of the keratoconus is still not known , despite extensive studies (e.g. long-term studies in the USA with 40,000 affected people).
Ultrastructural studies show that the regular layering of the collagen lamellae in the cornea is broken. The arrangement of the collagen lamellae in an orthogonal layer is destroyed. The destruction probably takes place via proteolytic activity. A genetic disposition is known in 23% of cases ; the only possible gene is VSX1 (Visual system homeobox gene 1; KTCN1). Excessive rubbing on the eye and certain environmental factors etc. can also be considered. The subsequent biomechanical imbalance between pressure load ( intraocular pressure ) and decreasing tissue strength then maintains a cycle that is expressed in the progression of the disease.
course
The change in the surface of the cornea can often be compensated for with glasses in the beginning, if the keratoconus is not very pronounced. Some patients get along well with glasses for a very long time. At this stage, some patients also have several glasses with different strengths and visual axes, some of which are worn in combination with contact lenses , as the visual strength and axis can change over the course of days.
If the keratoconus progresses and the cornea changes significantly, the ametropia can usually be reduced with dimensionally stable contact lenses.
If sufficient vision can no longer be achieved even with contact lenses because the cone is very far advanced or the contact lenses can no longer be adjusted well, the defective cornea can be exchanged for a transplant. However, this only occurs in around 20% of cases. The transplant is carried out in an eye clinic. There the defective cornea is exchanged for a donor cornea or, more rarely, stabilized with other methods.
Stages
Marc Amsler
Marc Amsler (1891–1968) divided keratoconus into four stages as early as 1950 . Here is the extended table by Dieter Muckenhirn with the corneal eccentricity that has been added since the sagittal radius measurement (with an ophthalmometer).
| Degree | Amslerwinkel | Central radii | Visus goggles | VisusCL 1 | transparency | Thickness HH 2 | Exc. 3 |
|---|---|---|---|---|---|---|---|
| 1 | 0 ° - 3 ° | > 7.5 | 1.0-0.8 | > 1 | normal | 0.5 mm | <0.8 |
| 2 | 4 ° - 9 ° | 7.5 - 6.5 | 0.8-0.2 | 1.0-0.8 | normal | 0.5 mm | <0.8 |
| 3 | > 9 ° | 6.5-5.8 | 0.2-0.1 | 0.8-0.4 | slightly cloudy | 0.25 mm | 1.2-1.5 |
| 4th | not measurable | <5.8 | <0.1 | 0.4-0.2 | very cloudy | <0.2 mm | > 1.5 |
1 CL = contact lens 2 HH = cornea 3 exc. = Excavation, in anatomy a space is called that spreads like a bulge between other tissue structures.
Jörg Krumeich
The stage is determined when one of the respective characteristics applies. The corneal thickness refers to the thinnest measured part of the cornea.
Conical stages according to Krumeich:
| stage | Clinical Criteria |
|---|---|
| Stage 1 | |
| Stage 2 |
|
| Stage 3 |
|
| Stage 4 |
|
Effects
- gradual deterioration in vision
- blurred vision
- Distortions
- Double or multiple images
- Ghosting when seeing
- Permanent red eyes
- Fatigue and tension in the facial muscles
- Severe inconvenience in dry, cold, dusty and stuffy air
- Extreme sensitivity to glare and light
- Halos
- Decreased twilight and night vision
- Loss or regular slipping of contact lenses
- Stars when looking at individual light sources
- Streaks when reading letters
These side effects do not have to occur in all people affected. The phenomena are individual, just as the development of keratoconus differs from eye to eye.
Comorbidities
Every second patient suffers more or less from hypersensitivity. Examples for this are:
- Dry eyes
- dark circles under the eyes
- Strong sensitivity to light
- Soft tissue rheumatism
- Skin problems ( atopic eczema , neurodermatitis , keratosis pilaris (grater skin))
- Allergies ( house dust , pollen , hay fever )
- asthma
- Vernal keratoconjunctivitis (conjunctivitis)
- Hypothyroidism
- Vitreous bone disease
- Multiple sclerosis (autoimmune disease of the CNS)
- Collagenoses (connective tissue disease)
- Retinopathia pigmentosa (a disease of the retina)
- Mitral valve prolapse (a congenital heart disease)
Hereditary diseases
An association (link) with various hereditary diseases is often observed:
- Trisomy 21
- Monosomy X Syndrome
- Ehlers-Danlos Syndrome
- Marfan's Syndrome
- Alport syndrome
- Silver-Russell syndrome
- Noonan's Syndrome
- Mulvihill-Smith syndrome
- Urrets-Zavalia Syndrome
- Floppy eyelid syndrome
The quality of tears is often impaired by such a secondary illness and can be subject to additional changes due to medication .
diagnosis
When a suspected keratoconus should retinoscopy be performed at irregular reflection (scissors phenomenon) already have an increased suspicion. A keratoscope (Placido disk) and a keratometry to check the corneal topography should be used for further examination.
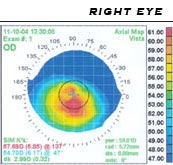
Diagnostic equipment
- Retinoscope , the oldest measuring device, with keratoconus the typical “fish mouth effect” can be seen
- Ophthalmometer (keratometer) for measuring corneal radii and adapt to contact lenses.
- Slit lamp , also called a corneal microscope , for recognizing the corneal layers and thickness
- Keratograph / Placido disk (video keratometer), for recording the surface structure
- Pentacam (Scheimpflug camera ) and OrbScan for recording the topography of the front and back ( endothelium ), as well as calculating the thickness of the cornea
- Optical coherence tomography (OCT) to record a cross section of the anterior segment of the eye, corneal thickness and the course of the surface can be documented
Demarcation
Particularly in the early stages, keratoconus can be mistaken for irregular corneal curvature or, due to inexperience, with pellucid marginal corneal degeneration (PMD). Other corneal degenerations that also cause thinning are the keratoglobus (affects the entire cornea). The keratectasia may be an initial stage of keratoconus.
Under Keratoconus posticus a curvature increase in the posterior cornea is understood the same time the corneal surface remains intact. There appears to be a link between keratoconus and keratoconus posticus.
With Fuchs endothelial dystrophy, however, the cornea thickens centrally.
treatment
glasses
One possibility for treatment is glasses , which are usually used at the beginning of the disease. Some ophthalmologists believe this option is better than contact lenses because contact lenses can trigger or at least make keratoconus worse. However, there is no evidence of this. Others report that treatment with hard contact lenses prevents further development of the corneal cone. There should even be a flattening of the cone tips.
It should be helpful to wear raster glasses with small holes in the lens. Through this point grid, the incident light beam is bundled and aligned in front of the eye. A cure or regression of the keratoconus cannot be achieved with this, however, there is no scientific proof of the effectiveness of the pinhole glasses.
contact lenses
Overview
| method | cases |
|---|---|
| soft lenses (standard) | only exceptional cases, in the early stages |
| soft special lenses | Alternative to dimensionally stable lenses 1 |
| dimensionally stable lenses | Means of choice |
| Scleral lenses (new generation) | Alternative if the eyes are very sensitive 1 |
| Duo system (piggyback system) | only in rare cases 2 |
1 If there is too much pressure on the cornea and / or if the skin is too sensitive to dust
Soft lenses
In the early stages, soft lenses may be used for correction. At a later stage, however, this is no longer possible, as the lenses do not adapt to the cone and so no vision improvement is possible. Hard contact lenses should be adjusted here because the tear fluid in connection with the contact lens compensates for the unevenness of the cornea. In later stages, however, this can become increasingly difficult. A Kerasoft IC contact lens can be fitted for mild or moderate keratoconus. This is more gas-permeable than conventional soft contact lenses. It is difficult to adjust this with an advanced cone.
Soft lenses (piggyback system)
In some cases of intolerance to wear comfort with dimensionally stable contact lenses, special soft keratoconus contact lenses or soft, highly oxygen-permeable contact lenses can now be worn as carrier lenses (protective lenses) under the hard contact lens (piggyback system).
Dimensionally stable lenses (hybrid lenses)
Dimensionally stable contact lenses are mostly used when glasses or soft lenses are no longer able to achieve adequate correction of the visual acuity, or when several images are seen due to corneal deformations.
The more the tip of the cornea, called the apex, bulges over time, the more the contact lens has to be curved, because the apex tip must not experience any pressure. At this advanced stage, special keratoconus lenses are individually fitted. These usually have to have a quadrant- specific shape in order to have a good and stable fit on the eye.
If the cone is very advanced, however, the image quality can deteriorate and the affected patient can no longer achieve 100% visual acuity. If the cone becomes more acute, a point is finally reached at which the normal dimensionally stable contact lens is no longer an effective treatment method. Be it that it can no longer hold, or that there is no longer any improvement in visual acuity, or that the lens has pressure points on the cornea. On the one hand, this is painful for the wearer, on the other hand, it is also not without risk, since the cornea is already thinned due to the keratoconus and damage can occur due to additional stress.
Then a corneal transplant must be considered. However, this only occurs in about 20% of all cases. Most patients get along well with contact lenses for a lifetime.
If even with dimensionally stable keratoconus lenses sufficient visual acuity cannot be achieved or these cannot be tolerated, scleral lenses or mini- scleral lenses can also be adapted. These cover the entire cornea and lie on the dermis.
The so-called hard-soft hybrid contact lenses represent a further possibility. They consist of a hard, dimensionally stable, oxygen-permeable core and a soft casing. These increase wearing comfort and reduce the irritation and intolerance that are often caused by the small, dimensionally stable lenses. Loss of a lens straight out of the eye is also less likely.
Scleral lenses
Special lenses
Janus lenses have a stable core and a soft outer area. Since these contact lenses are very complex to manufacture, they have never really caught on. This type of lens is not very gas permeable, so that the cornea is not adequately supplied with oxygen. Therefore, these lenses should only be used in special cases.
In rare cases (with high sensitivity or greatly increased sensitivity to dust) a “ piggyback system ” can also be used, in which a hard contact lens is fitted to a soft lens.
For patients with intolerance, or those who have problems in a dusty environment, there is a new supply option with HydroCone contact lenses (soft keratoconus lenses) to increase visual performance and comfort.
Operations
The aim of surgical treatments is visual rehabilitation (improvement of vision) and stopping the further development of the disease. The only effective method to date is cross-linking .
CrossLinking (CXL)
The ophthalmologist Theo Seiler is considered the discoverer and developer of crosslinking (collagen injury) , who also performed the first laser-guided radial cuts (RK / Radial Keratotomy ) of the cornea to eliminate myopia with the excimer laser . The effectiveness of this therapy in stopping the progression of keratoconus and thus possibly avoiding a corneal transplant has been proven in many studies. Theo Seiler has been honored by many international ophthalmological societies for his services to the treatment of keratoconus and is a welcome speaker at national and international congresses. Over the past 10 years, many different approaches have been clinically tested and different treatment protocols have been compared. Crosslinking prevents progression and is now also recommended by statutory health insurers in stages I and II. It is the only treatment method so far that has been proven to be able to stop the progression of keratoconus for several years. It may even be effective over the long term. Crosslinking has hardly any effect on a small number of those affected. The photochemical process mediated by riboflavin and ultraviolet radiation (UVA) cross-links the collagen of the cornea with one another, thus achieving a stabilizing effect. In order to be able to perform CXL, a corneal thickness of at least 400 µm is required. After the biomechanical stiffening of the cornea, a topographically guided transPRK or standard PRK is to be carried out with the excimer laser in order to return the cornea to an optimal, optically more effective contour and thus also to improve visual acuity. There are also treatment protocols that carry out the topographically guided PRK with subsequent crosslinking in one session. However, the simultaneous interaction of the two treatments seems to have a considerably stronger effect on the transparency of the cornea ("haze" formation) than a crosslinking treatment alone, whose low risk of haze formation has meanwhile been proven in the literature.
Whether the obvious successes of the crosslinking treatment justify doubts about worsening the prognosis of a corneal transplantation is doubted and remains to be seen.
Intracorneal Implants
Before a corneal transplant is performed in very advanced cases, intracorneal implants can be used to achieve visual rehabilitation and, in many cases, delay or even prevent the need for a corneal transplant.
ICRS - Intracorneal Ring Segments
An intrastromal corneal ring segment (ICR or ICRS or Intacs) is an implant for the cornea of the eye. The ICRS have been in use since 1996. The ICR consists of two small ring segments that together have an inner diameter of 6.7 mm. These transparent plastic segments are made of the plastic polymethyl methacrylate (PMMA), the same material that has been implanted as a lens replacement in cataracts for around 30 years . They are pushed into (intra) the layers ( stroma ) of the cornea at the edge of the cornea, thereby flattening the central cornea. The tunnel incision required for this can be prepared with a blunt knife or with the femtosecond laser . Depending on the ring size, myopia can be between −1 dpt. and −4 dpt. Getting corrected. A corneal curvature should not be present.
The surgery should only be performed by an experienced corneal surgeon. The risk of a cutting error can be reduced by using the femtosecond laser. In the event of an over or under correction, the ring segments can in principle be exchanged or removed again. Because of the radial incision in the cornea, however, the wound must always be closed with a suture. A removal or an exchange can therefore only take place via the opening of the already scarred wound, and this must then be sutured again.
The ICRS are mostly used for visual rehabilitation of keratoconus. However, the segments have ends and can therefore often lead to pressure atrophy of the corneal tissue as a complication, which causes the segments to “grow out” of the corneal surface, which is referred to as “extrusion” or “corneal melting”.
CISIS (MyoRing)
A full ring implant ( MyoRing ) is inserted into the cornea to treat keratoconus. The treatment method is called CISIS (Corneal Intrastromal Implantation Surgery) and has been approved since around 2007. The ring is inserted into the cornea between an anterior and posterior corneal lamella in a virtual gap (closed corneal pocket). Because of the lamellar structure of the cornea, the corneal pocket is biomechanically neutral. The procedure can be combined with crosslinking in one treatment session. No seams are necessary. The MyoRing implantation can replace a corneal transplant in many cases. CISIS can no longer be used if the corneal thickness is less than 350 micrometers or the central K values are above 60 diopters. The latest results show that the MyoRing not only leads to visual rehabilitation, but can also stop the progression of keratoconus because of its closed construction.
Corneal transplant (keratoplasty)
Contact lenses may not be tolerated if, for example, the eye does not produce enough tear fluid. Then, despite a possibly better correction, a surgical intervention must be considered. Otherwise, a transplant is only carried out if sufficient visual acuity is no longer achieved with contact lenses (visual acuity below 0.3) or treatment with intra-corneal implants is no longer possible.
During a keratoplasty , the cornea is punched out so that only a small margin remains ( trepanation ). The cut-out piece is replaced by donor tissue and sewn with a double seam to make it watertight. The Heidelberg University Eye Clinic is the first in Germany to use a new laser device for corneal transplants, which may make it possible to dispense with sutures in the future. There is a lamellar (layered) keratoplasty and a perforating (penetrating) keratoplasty.
The goal must always be to preserve your own cornea for as long as possible. The healing process after a transplant can take up to two years and even after a transplant, around 85–90% of those affected have to wear dimensionally stable, mostly special contact lenses again. A hasty keratoplasty is not advisable, the alternatives listed below may also help. Most of them are relatively new or there are no long-term studies. When it comes to the question of which method makes sense in which stages, there are only guidelines, but the attending physician can best assess when which type of keratoplasty can help.
Alternatives
In addition to the methods mentioned, there are also other methods that are not discussed further here:
- CKT Circular Keratotomy
-
Verisyse (Artisan) lens
- The refractive epikeratophakia (EPI) is applied in stage II and III
- Keraform treatment
- conditionally with an excimer laser .
-
Keratoplasty
- Perforating keratoplasty in the IV stage
- Deep anterior lamellar keratoplasty (TLKP): "Deep anterior lamellar keratoplasty" (DALK), for advanced keratoconus
-
Keratotomy
- Mini-Asymmetric Radial Keratotomy (MARK) designed by Marco Abbondanza. Stage I and II.
- Radial asymmetric keratotomy (ARK or mini ARK) performed by Professor Massimo Lombardi. Although Lombardi has used this treatment since 1993, no scientific studies have been carried out to date.
- Bowman Layer Transplant for advanced keratoconus
Laser treatment ( PRK , LASIK ) is contraindicated .
Other treatments such as hormone therapy , using vitamin D complexes or vitamin E as well as topical cortisone have not shown any sure success.
Prophylaxis (prevention)
According to all that is known so far, one cannot actively protect oneself against keratoconus, since the disease probably has at least one genetic disposition as an additional cause.
In general, it certainly makes sense to avoid anything that puts a lot of strain on the eyes and cornea. This is especially the "rubbing of the eyes", which is probably caused by too little tear fluid or too frequent screen work. Staying in rooms with dusty, smoky air or air from air conditioning systems is perceived by many as a burden.
In any case, it is an advantage to drink a lot, to exercise in the fresh air and to avoid smoky or dusty surroundings.
Many practitioners and those affected are also of the opinion that wearing contact lenses is a very significant burden on the cornea of the eye and should be avoided as far as possible. To date, it has not been proven by studies that wearing contact lenses promotes keratoconus.
Reimbursement of costs (specific to Germany)
Since the health reform of 2004, it has proven difficult for health insurance companies to cover the costs of contact lenses . It is particularly difficult to get coverage in the early stages of fitting keratoconus lenses. Many of the alternatives mentioned above are also not paid for, as long-term studies are usually lacking or the surgical methods are controversial.
The current (2013) aids directory of the National Association of Statutory Health Insurance Funds lists so-called keratoconus lenses (custom-made) in the category "Optically corrective special lenses" , the costs of which are covered up to an agreed fixed amount under a corresponding medical indication.
According to a press release by the Federal Joint Committee on June 19, 2014, it was examined whether a treatment of keratoconus by means of UV crosslinking with riboflavin must be covered by the statutory health insurance .
The Federal Joint Committee (G-BA) decided on July 19, 2018 to include UV cross-linking with riboflavin in keratoconus in the catalog of services of the statutory health insurance. The decision came into force on October 12, 2018 after publication in the Federal Gazette ( BAnz AT October 11 , 2018 B2 ). On March 29, 2019, the Joint Evaluation Committee determined the compensation in the EBM. This means that the statutory health insurance companies can now offer their members this service, more than 20 years after the method was invented.
forecast
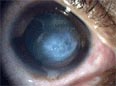
A prognosis for the course of the keratoconus is not possible because neither the causes of the disease nor the environmental influences on the course have been adequately investigated.
Some sufferers need a transplant after a short time, for example weeks or months after the first onset of the disease, while others can cope with glasses or contact lenses for decades or permanently.
The only observation that is generally confirmed by those affected and the practitioners is the experience that keratoconus often begins between the ages of 15 and 20 and often comes to a standstill between the ages of 40 and 50.
Problems
everyday life
A big problem is that lenses are always lost and have to be bought or applied for from health insurance companies. The eyes are also often overstimulated (reddening, extreme tearing, etc.) then only the removal of the contact lenses helps. The ophthalmologist must clarify whether there is any impairment in driving a motor vehicle .
Visual acuity can fluctuate several times a day. These changed visual conditions can lead to a deterioration in thought, memory and concentration processes.
Since keratoconus and its peculiarities are still relatively unknown in the population, explanations may be required (Why do you have to wear contact lenses and no glasses? Why do you see poorly today and good tomorrow? Why cannot you work just because the contact lens has been lost is? ...)
Driving license
According to a recommendation by the German Ophthalmological Society (DOG) and the Professional Association of Ophthalmologists in Germany (BVA), an annual follow-up examination is required for road traffic . A re-assessment is only possible after two years.
Ticket
A class 2 medical certificate can only be issued through an examination by the Luftfahrt-Bundesamt or the responsible body. The minimum requirements are that the required visual performance is met with a visual aid and that regular checks are carried out. The intervals are determined by aero-medical centers or aero-medical experts.
police
In Germany, the state police , federal police , SEK , MEK and GSG have 9 exclusion criteria for contact lens wearers and for laser eye operations in the last 12 months. Sufficient eyesight even without glasses is required. The BKA demands that 100% vision aid must be seen with the good eye and at least 80% must be seen with the poor eye. After an operation you have to wait 12 months and the corneal thickness is sufficient to be able to work. Far-sightedness over 2.5 dpt. must not be present. The uncorrected visual performance must not be below 50% until one is 20 years old or below 30% from the age of 20. Further information on suitability for use can also be found in Police Service Regulation 300 (PDV 300) - Medical Assessment of Fitness for Police Service and Police Service Capacity (2012 edition).
military
- In the Bundeswehr , keratoconus is given as grade VI (see: ZDv 46/1 Annexes 3.1 and 3.2). This means that military capability can be permanently excluded, the fitness level is therefore T5 (not fit for military service).
- The soldier can be drafted to the Austrian Armed Forces after the position (draft) despite keratoconus.
- In the Swiss Army , the soldier is unfit, unless the ametropia is operated on and certified as good after a medical certificate.
See also
literature
- A. Bochert, J. Berlau, D. Koczan, B. Seitz, HJ Thiessen, R. Guthoff: Gene expression in keratoconus. first use of DNA microarrays. . In: The ophthalmologist. 7/2003, ISSN 0941-293X .
- John W. Chandler, Joe Sugar, Henry F. Edelhauser: Disease of the external eye - cornea, conjunctiva, sclera, eyelids, lacrimal system . Urban & Fischer, Munich, 1999, ISBN 3-86126-129-4 .
- C. Edmund: Corneal topography and elasticity in normal and keratoconic eyes: A methodological study concerning the pathogenicity of keratoconus. In: Acta Ophthalmol Suppl. Volume 193, 1989, pp. 1-36. (English).
- Nathan Efron: Cornea . Butterworth Heinemann, 2001, ISBN 0-7506-4798-1 (English).
- R. Eschmann, D. Roth-Muff: The keratoconus in the subclinical stage. In: NOJ. 2/1994.
- Achim Langenbucher, Gabriele C. Gusek-Schneider, Murat M. Kus, Berthold Seitz: Topography-based calculation of the keratoconus dimensions. In: Ophthalmology. 214/1999, pp. 372-377.
- James N. Parker, Philip M. Parker: The Official Patient's Sourcebook on Keratoconus. Icon Health, 2002, ISBN 0-597-83187-4 . (English).
- Anne Christina Preclik: Long-term course after perforating keratoplasty for keratoconus - effects of preoperative vision and astigmatism on functional results . (PDF; 235 kB) Dissertation . Friedrich-Alexander University, Erlangen-Nuremberg 2010.
- O. Stachs, T. Gerber, D. Koczan, U. Sommer, H.-J. Thiessen, R. Guthoff: The structure of the extracellular matrix in keratoconus. In: The ophthalmologist. 09/2002 and 04/2004.
- Theo Seiler: Refractive Surgery of the Cornea . Georg Thieme Verlag, 2000, ISBN 3-13-118071-4 .
- Karl Velhagen : The ophthalmologist . Volume 3.2: (tear organs, eyelids, orbit, conjunctiva, cornea, dermis, lens, vitreous humor, aqueous humor) 1975.
- Irina Wahrendorf: Difficulty in life and lifestyle for people with the visual impairment keratoconus. www.wahrendorf-kontaktlinsen.de, 2004.
- Karla Zadnik, Joseph T. Barr: Diagnosis Contact Lens Prescribing, and Care of the Keratoconus Patient: Clinical Practice in Contact Lenses . Butterworth-Heinemann, 1999, ISBN 0-7506-9676-1 . (English)
- Karla Zadnik, Joseph T. Barr: Keratoconus - A Medical Dictionary, Bibliography, and Annotated Research Guide to Internet References . ICON Health Publications, 1999, ISBN 0-497-00623-5 . (English)
- Center for Contact Lens Research, School of Optometry, University of Waterloo, Canada: Correcting keratoconus with dimensionally stable contact lenses , ( Appendix )
- Clinical monthly sheets for ophthalmology. Volume 211, Issue 3, September 1997.
- New treatment options for keratoconus . (PDF; 792 kB)
- Keratoconus - an information sheet for those interested and affected. (PDF; 562 kB)
- Keratoconus. (PDF; 306 kB)
- Medicine: plastic rings against keratoconus. Netnews, August 29, 2005
Web links
- Global Keratoconus Foundation (English)
- National Keratoconus Foundation (multilingual)
- About Keratoconus Eye Disease and Hybrid Contact Lens. Keratoconus page from the keratoconus hybrid lens manufacturer Synergeye (English)
- About Keratoconus Eye and Soft Keratoconus Contact Lens. (PDF; 73 kB) Page on keratoconus from the special lens manufacturer SwissLens
- Atlas of Ophthalmology images of the various stages of keratoconus
Individual evidence
- ^ A b P. Caroline, M. Andre, B. Kinoshita, J. Choo: Etiology, Diagnosis, and Management of Keratoconus: New Thoughts and New Understandings. (No longer available online.) In: Pacific University College of Optometry. Archived from the original on January 7, 2009 ; Retrieved December 15, 2008 .
- ^ J. Nottingham: Practical observations on conical cornea: and on the short sight, and other defects of vision connected with it. J. Churchill, London 1854.
- ↑ W. Bowman: On conical cornea and its treatment by surgery. In: Ophthalmic Hosp Rep and JR Lond Ophthalmic Hosp. Volume 9, 1859, p. 157.
- ↑ JF Horner: For the treatment of keratoconus. In: Clinical monthly sheets for ophthalmology. 1869.
- ↑ a b R. Rochels: Acute Keratoconus in Down's syndrome. In: Albrecht von Graefes Archive for Clinical and Experimental Ophthalmology. Volume 212, 1979, pp. 117-128, doi: 10.1007 / BF00587603 .
- ^ W. Messikommer, University Eye Clinic Zurich: Acute keratoconus in a child . In: Ophthalmologica. Volume 123, 1952, pp. 326-329.
- ↑ A. Daxer, P. Fratzl: Collagen fibril orientation in the human corneal stroma and its implications in keratoconus. In: Invest Ophthalmol Vis Sci. 38, 1997, pp. 121-129.
- ↑ Keratoconus 1; KTCN1. In: Online Mendelian Inheritance in Man . (English)
- ^ A. Daxer: Biomechanics of the cornea. In: International Journal of Keratoconus and Ectatic Corneal Diseases. Volume 3, 2014, pp. 57-62.
- ↑ Clinical classification of keratoconus according to Dr. Crumbly.
- ↑ Clinical Monthly Ophthalmology. Volume 211, 1997, pp. 94-100.
- ^ Journal of Refractive Surgery. September 2008.
- ↑ B. Seitz: Keratoconus and Thyroid . In: Ophthalmological News. 09.2012, DOG Congress Edition 1, 22
- ↑ B. Seitz: ( Page no longer available , search in web archives: Keratoconus keratometer values and thyroid dysfunction in keratoconus. ) (PDF; 262 kB) 2012.
- ↑ P. Biro, DA Vagts, U. Emmig, T. Pasch: Anesthesia for rare diseases. Springer-Verlag, 2010: ISBN 978-3-642-01046-0 . (books.google.de)
- ↑ C. Grünauer-Kloevekorn, G. Duncker: Keratoconus: Epidemiology, Risk Factors and Diagnostics. In: Clinical monthly sheets for ophthalmology. Volume 223, 2006, pp. 493-502, doi: 10.1055 / s-2005-859021 .
- ↑ Wikibooks: Collection Medicine: Eye
- ↑ Keratoconus - The Mystery of Pathogenesis. In: Clinical monthly sheets for ophthalmology. 1986, p. 365 f.
- ↑ Stefan Rau, Gernot Duncker: Keratoconus in Mulvihill-Smith syndrome. In: Clinical monthly sheets for ophthalmology. Volume 205, 1994, pp. 44-46, doi: 10.1055 / s-2008-1045490 .
- ↑ EyeWiki: Urrets-Zavalia Syndrome
- ↑ ED Donnenfeld, HD Perry, RP Gibralter, HJ Ingraham, IJ Udell: Keratoconus associated with floppy eyelid syndrome. In: Ophthalmology. Volume 98, Number 11, November 1991, pp. 1674-1678. PMID 1800928 .
- ↑ Karsten Bronk: The adaptation of dimensionally stable contact lenses. (PDF; 380 kB)
- ↑ Katrin Warken: Measurement method for determining the peripheral corneal radii. (PDF; 334 kB). (= Galifa moment. 05/2009).
- ↑ ( page no longer available , search in web archives: Topographie - the whole cornea )
- ↑ Michael W. Belin, Renato Ambrósio Jr., Andreas Steinmueller: The principle behind keratoconus early detection according to Belin / Ambrosio. 2009.
- ↑ Help Keratoconus: Orbscan, Keratoconus ( Memento from September 24, 2012 in the Internet Archive )
- ↑ subclinical keratoconus
- ↑ Lisa Müßig: Screening methods for keratoconus . 2014.
- ↑ Corneal wavefront analysis to differentiate between keratoconus and pellucid marginal degeneration (PDF; 165 kB)
- ↑ Nisha Garg, Ta C. Chang, Bibiana Jin Reiser, Kara M. Cavuoto: A novel characterization of posterior keratoconus using anterior segment optical coherence tomography in an infant: a case report . In: BMC Ophthalmol . No. 15 , November 4, 2015, p. 158 , doi : 10.1186 / s12886-015-0139-3 , PMC 4634598 (free full text).
- ↑ Varsha M Rathi, Preeji S Mandathara, Srikanth Dumpati: Contact lens in keratoconus . In: Indian J Ophthalmol . tape 61 , no. 8 , 2013, p. 410-415 , doi : 10.4103 / 0301-4738.116066 , PMC 4631016 (free full text).
- ^ Center for Contact Lens Research, School of Optometry, University of Waterloo, Canada: Correcting keratoconus with dimensionally stable contact lenses ( Memento of September 7, 2012 in the Internet Archive ).
- ↑ G. Wollensak, E. Spoerl, T. Seiler: Riboflavin / ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus . In: Am J Ophthalmol. tape 135 , no. 5 , 2003, p. 620-627 .
- ↑ Lutz Bloomberg: Collagen crosslinking with riboflavin and UVA light instead of corneal transplantation. 2007. (PDF; 783 kB)
- ↑ UV crosslinking: corneal crosslinking with UVA radiation and riboflavin.
- ↑ Renata LB Morales et al.: Haze ans Visual Acuity Loss After Sequential Photorefractive Keratetomy and Corneal Cross-linking for Keratoconus . In: ISRS (Ed.): J Refract Surg . tape 35 , no. 2 . SLACK Incorporated, Thorofare NJ 2019, pp. 109-114 .
- ↑ T. Koller, M. Mlocken, T. Seiler: Complication and failure rates after corneal cross linking . In: ASCRS (Ed.): J Cataract Refract Surg . tape 35 . Elsevier, 2009, ISSN 0886-3350 .
- ^ JH Krumeich, B. Brand-Saberi, V. Chankiewitz, E. Chankiewitz, R. Guthoff: Induction of neoplasia after deep anterior lamellar keratoplasty in a CXL-treated cornea. In: Cornea. Volume 33, number 3, March 2014, pp. 313-316, doi: 10.1097 / ICO.0000000000000047 . PMID 24452218 .
- ↑ a b C. Ferrer et al .: Causes of intrastromal corneal ring segment explantation: clinicopathologic correlation analysis. In: Journal of Cataract and Refractive Surgery . Volume 36, 2010, pp. 970-977.
- ↑ J. Colin et al .: Correcting keratoconus with intracorneal rings. In: Journal of Cataract and Refractive Surgery. Volume 26, 2000, pp. 1117-1122.
- ↑ K. Jaddidi et al: Complications of instrastromal corneal ring implantation (Keraring 355 °) using a Femtosecond Laser for Channel creation. In: International Journal of Keratoconus and Ectatic Corneal Diseases. Volume 3, 2014, pp. 53-56.
- ↑ A. Daxer, H. Mahmoud, RS Venkateswaran: Intracorneal continuous ring implantation for keratoconus: One year follow-up. In: J Cataract Refract Surg. 36, 2010, pp. 1296-1302.
- ↑ A. Daxer: Adjustable Intracorneal Ring in a Lamellar Pocket for Keratoconus. In: Journal of Refractive Surgery . Volume 26, 2010, pp. 217-221.
- ^ A. Daxer, H. Mahmoud, RS Venkateswaran: Corneal Crosslinking and Visual Rehabilitation in Keratoconus in One Session Without Epithelial Debridement: New Technique. In: Cornea. Volume 29, 2010, pp. 1176-1179.
- ↑ Pavel Studeny, Deli Krizova, Zbynek Stranak: Clinical Outcomes after Complete Intracorneal Ring Implantation and Corneal Collagen Cross-Linking in an Intrastromal Pocket in One Session for Keratoconus. In: Journal of Ophthalmology. 2014, p. 1, doi: 10.1155 / 2014/568128 . PMC 4172981 (free full text)
- ↑ H. Mahmoud, RS Venkateswaran, A. Daxer: Implantation of a complete ring in an intracorneal pocket for keratoconus. In: Journal of Refractive Surgery. Volume 27, 2011, pp. 63-68.
- ↑ A. Daxer: MyoRing for Central end Noncentral Keratoconus. In: International Journal of Keratoconus and Ectatic Corneal Diseases. Volume 1, 2012, pp. 117-119.
- ↑ M. Jabbarvand, A. Salamatrad, H. Hashemian, M. Khodaparast: Continuous corneal intrastromal ring implantation for treatment in keratoconus in an Iranian population. In: American Journal of Ophthalmology. Volume 155, 2013, pp. 837-842.
- ↑ A. Daxer: Corneal thickness after MyoRing implantation for keratoconus. In: International Journal of Keratoconus and Ectatic Corneal Diseases. Volume 3, 2014, pp. 15-19.
- ↑ MyoRing Treatment of Keratoconus. cisis.com
- ↑ A. Daxer, A. Ettl, R. Hörantner: Long-Term Results of MyoRing Treatment of Keratoconus. In: Journal of Optometry. Volume 10, No. 2, 2017, pp. 123-129.
- ↑ A. Prangl, A. Ettl, R. Hörantner, A. Daxer: Individual long-term visual stability after MyoRing treatment of keratoconus. In: International Journal of Keratoconus and Ectatic Corneal Diseases. Volume 5, 2015, pp. 53-56.
- ↑ J. Krumeich, J. Daniel: Lebend-Epikeratophakie and deep lamellar keratoplasty for the stage-appropriate surgical treatment of keratoconus (KK). In: Ophthalmology. Volume 18, 1997, pp. 351-362.
- ^ Laser Vision Center: Epikeratophakia
- ↑ University Hospital Düsseldorf: Various Keratoplasty Techniques ( Memento from February 24, 2014 in the Internet Archive )
- ↑ Keratoplasty: Indications and Techniques
- ↑ Deep anterior lamellar keratoplasty (DALK) using the "big bubble" technique for the treatment of advanced keratoconus. In: Ophthalmology. September 1, 2011.
- ↑ Perspectives of deep anterior lamellar keratoplasty ( Memento from February 22, 2014 in the Internet Archive ) In: Der Ophthalmologe. 2011.
- ↑ M. Lombardi, M. Abbondanza: Asymmetric radial keratotomy for the correction of keratoconus. In: Journal of Refractive Surgery . Volume 13, Number 3, 1997 May-June, pp. 302-307. PMID 9183763 .
- ↑ Mini cheratomia radiale asimmetrica (mini ARK) per la correzione chirurgica del cheratocono in fase iniziale, nell'ipermetropia e nelle miopie lievi. ( Memento of December 20, 2013 in the Internet Archive ) centronazionalelaser.com
- ↑ Felicita Donalisio: La curva pericolosa della cornea. ilgiornale.it
- ↑ M. Kohlhaas, J. Draeger, A. Böhm, M. Lombardi, M. Abbondanza, M. Zuppardo, M. Görne: On the aesthesiometry of the cornea after refractive corneal surgery. In: Clinical monthly sheets for ophthalmology. Volume 201, 1992, p. 221, doi: 10.1055 / s-2008-1045898 .
- ^ Jack Parker: Bowman layer transplantation effective in advanced keratoconus. (No longer available online.) In: Ophtalmology Times. November 1, 2016, archived from the original on May 1, 2017 ; accessed on May 9, 2017 .
- ↑ Keratoconus and Related Changes . Accessed August 18, 2013.
- ↑ List of resources of the National Association of Statutory Health Insurance Funds
- ↑ Federal Joint Committee , June 19, 2014: Corneal disease of the eye: G-BA examines treatment method , accessed on June 12, 2015.
- ↑ Federal Joint Committee, June 19, 2014: Initiation of the advisory procedure: Evaluation of UV crosslinking with riboflavin in keratoconus in accordance with Section 135 (1) SGB V , accessed on June 12, 2015.
- ↑ [N15-05] UV crosslinking with riboflavin in keratoconus. IQWiG . February 5, 2016, accessed March 27, 2016.
- ↑ Guideline methods of contract medical care: UV crosslinking with riboflavin in keratoconus - Federal Joint Committee. Federal Joint Committee, accessed on August 11, 2018 .
- ↑ Assessment of fitness to drive for road traffic 2013 - DOG ( Memento from November 23, 2015 in the Internet Archive )
- ↑ The 98 Disqualification Criteria
- ↑ Air traffic JAR-FCL
- ↑ Examination of suitability for police service . Retrieved May 2, 2017.
- ↑ Federal Police Academy: Information sheet from the Police Medical Service of the Federal Police for applicants about the police medical examination.Retrieved : May 2, 2017.
- ↑ sek-Einsatz: Police training - requirements for recruitment Accessed: May 2, 2017.
- ↑ BKA: Information sheet of the medical service of the Federal Criminal Police Office for applicants for the federal police enforcement service. Accessed: May 2, 2017.
- ↑ Table of health numbers and digits (for conscript and civil service)
- ↑ Table of health numbers and digits (only for regular and professional soldiers)
- ↑ ZDv 46/1 Annex 1/1: Definitions, explanations
- ↑ Federal Chancellery, February 24, 2005: VwGH 2003/11/0308
- ^ Civil doctor - military service III