Verapamil
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
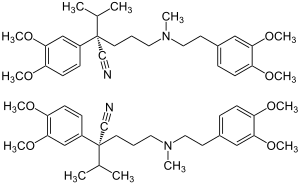
| 1: 1 mixture of ( S ) -form (top) and ( R ) -form (bottom) | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Verapamil | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 27 H 38 N 2 O 4 (verapamil) | |||||||||||||||||||||
| Brief description |
light yellow, viscous oil |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 454.60 g · mol -1 (verapamil) | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
138.5–140.5 ° C (verapamil hydrochloride, decomposition) |
|||||||||||||||||||||
| boiling point |
243-246 ° C (1.33 Pa ) |
|||||||||||||||||||||
| pK s value |
8.6 (verapamil hydrochloride) |
|||||||||||||||||||||
| solubility |
Verapamil: insoluble in water (4.47 mg · l −1 at 25 ° C), soluble in benzene , diethyl ether , very easily soluble in lower alcohols , acetone and chloroform |
|||||||||||||||||||||
| Refractive index |
1.5448 (25 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Verapamil , more precisely verapamil hydrochloride, is a drug from the group of calcium antagonists or calcium channel blockers, which has a vasodilator effect and retards the conduction in the AV node of the heart . Verapamil belongs to class IV of the Vaughan / Williams classification of antiarrhythmics and is one of the phenylalkylamines. It is used to treat:
- of coronary heart disease ,
- heartbeat disorders ( arrhythmia ),
- of high blood pressure ,
- of cluster headache ( off-label use ) and
- hypertrophic cardiomyopathy ( off-label use ).
history
Around 1960, Knoll AG began researching a new coronary dilating substance. She used papaverine as the starting substance , the effect of which was already known. From this Ferdinand Dengel developed a group of similar compounds. From this he finally selected the substance D 365, which is now known as verapamil. In various studies, Hans Haas (1907–1996) and G. Härtfelder demonstrated its coronary dilating effect for the first time. However, at that time there was no knowledge of the exact mechanism of action. Nevertheless, it entered the German market in 1963 - without any economic success. For this reason, verapamil was postulated to have a β-receptor-blocking effect, which triggered heated discussions about the therapeutic principle of action. In November of the same year , the companies Knoll AG and Hoechst commissioned Albrecht Fleckenstein to investigate the specific mechanism of action of the two substances verapamil and prenylamine . Already in 1964 discovered Fleckenstein that the substances in the heart of a lack of calcium - ions cause. This was the hour of birth of the calcium antagonists . In 1967 Fleckenstein clearly differentiated the substances verapamil ( Isoptin® ) and gallopamil ( Procorum® ) from beta blockers and assigned them to the new substance class that officially got its name in 1969.
application
In patients with coronary artery disease, verapamil is used in chronically stable angina pectoris , in unstable angina pectoris and especially in vasospastic angina pectoris ( Prinzmetal's angina ), in patients after a heart attack only if there is no heart failure and beta blockers are not indicated.
As an antiarrhythmic for the treatment of cardiac arrhythmias , verapamil can be prescribed as a preventive measure against paroxysmal supraventricular tachycardias and with an initial dose of about 5 mg for at least two minutes in adults to slow the pulse rate in atrial fibrillation and atrial flutter with increased ventricular rate .
Verapamil is also used as a prophylaxis for cluster headache . Since a comparably high dose of 3–4 × 80 mg and increases of up to 960 mg / d (in individual cases even more) are required to suppress cluster headache attacks, regular ECG monitoring is recommended.
Verapamil is rarely used exclusively to lower blood pressure in arterial hypertension .
Verapamil is not approved for the treatment of induratio penis plastica in Germany.
Contraindications and warnings
Verapamil must not be used in shock , acute myocardial infarction with complications such as bradycardia , hypotension or left heart failure, pronounced conduction disorders such as SA or AV block II. And III. Grades, sinus node syndrome , overt heart failure, atrial fibrillation or atrial flutter in patients with WPW syndrome and known hypersensitivity to verapamil and in the first six months of pregnancy . Beta blockers (e.g. bisoprolol) are contraindicated when taking verapamil, as they can cause AV block.
Careful monitoring is required for Grade I AV block, hypotension (systolic <90 mmHg ), bradycardia (pulse <50 beats per minute), severely impaired liver function , myasthenia gravis , Lambert-Eaton syndrome, and advanced Duchenne muscular dystrophy .
In the last trimester of pregnancy, verapamil may only be used after carefully weighing the benefits and risks. Since it passes into breast milk , it should not be taken while breastfeeding.
Grapefruit can increase blood plasma levels when verapamil is taken at the same time by inhibiting the first-pass effect . Urinary drugs (water tablets, diuretics ) increase the blood pressure lowering effect. Concomitant use of verapamil and simvastatin in higher doses increases the risk of myopathy / rhabdomyolysis and the simvastatin dose should be adjusted accordingly. The intravenous application of verapamil must not be used in patients with simultaneous beta-blocker therapy (exception: intensive care medicine). If loperamide (an anti-diarrheal agent) and verapamil are taken at the same time , signs of respiratory depression can be triggered. Verapamil blood plasma values were found to be around 25% lower in smokers than in non-smokers.
Verapamil interacts with many drugs because it inhibits cytochrome P450 3A4 and P-glycoprotein . It is itself broken down by cytochrome P450 3A4.
chemistry
Stereoisomerism
Verapamil is chiral , so it contains a stereocenter. There are thus two enantiomers , the ( R ) form and the ( S ) form. The commercial products contain verapamil hydrochloride as a racemate (1: 1 mixture of enantiomers).
synthesis
A summary of the syntheses for verapamil, starting from 3,4-dimethoxyphenylacetonitrile and 2-chloropropane , is described in a review article. A variety of sources are available for the starting materials 3,4-dimethoxyphenylacetonitrile and N -methylhomoveratrylamine .
The principle of convergent synthesis, which has proven itself in natural product synthesis, is used to synthesize the final stage verapamil . H. two larger modules are linked to form the output stage. The modules are linked according to three strategies:
1.) N -alkylation of N -methyl-homoveratrylamine of the formula (I) with an alkyl chloride of the formula (II) (see reaction scheme ):
This route was also registered by the inventors for the synthesis of chiral verapamil.
2.) Reductive alkylation:
Patent applications for the reaction of 5-methylaminovaleronitrile derivative of formula (III) with 3,4-dimethoxyphenylacetaldehyde of formula (IV) were filed in 1973, for N -methyl-homoveratrylamine of formula (I) with a hexanal derivative of the formula (V) 1982 and for the hydrogenation of a glutaronitrile derivative of the formula (VI), which is obtained via cyanoethylation with methoxypropionitrile, in the presence of homoveratrylamine of the formula (I) 1984.
3.) Build-up of the quaternary carbon atom by C -alkylation:
Strong auxiliary bases are required for this. Isopropyl homoveratronitrile of the formula (VIII) is alkylated by 3-chloropropylamine of the formula (IX) in the presence of sodium amide . With phase transfer catalysis with powdered potassium hydroxide, the C-alkylation should also succeed.
Improved isolation and cleaning methods are claimed by two working groups in 1993 and 2016.
pharmacy
Trivia
Verapamil has been on the World Health Organization's Essential Medicines List since 1983 .
Trade names
Monopreparations
Falicard (D), Flamon (CH), Isoptin (D, A, CH), Veragamma (D), Vera-Lich (D), Veramex (D), Veranorm (D), Verapabene (A), Verasal (D ), Veroptinstada (D, A), various generics (D)
Combination
preparations Captocomp (A), Convit (A), Cordichin (D), Isoptin RR (D), Tarka (D, CH), Veracapt (A)
- Note: Isoptin RR is available as a monopreparation in Austria.
Web links
Individual evidence
- ↑ a b c d Entry on Verapamil. In: Römpp Online . Georg Thieme Verlag, accessed on May 29, 2014.
- ↑ a b The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals. 14th edition. 2006, ISBN 0-911910-00-X , p. 1710.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-518.
- ↑ a b data sheet (±) -Verapamil hydrochloride from Sigma-Aldrich , accessed on October 22, 2016 ( PDF ).
- ↑ Cluster headache and trigeminal autonomic headache. In: Hans-Christoph Diener (Hrsg.): Guidelines for diagnosis and therapy in neurology. 4th, revised. Edition. Published by the "Guidelines" commission of the German Society for Neurology. Georg Thieme Verlag, 2008, ISBN 978-3-13-132414-6 , pp. 567-572. Online version (PDF; 817 kB)
- ^ Wolf-Dieter Müller-Jahncke , Christoph Friedrich , Ulrich Meyer: Medicinal history . 2., revised. and exp. Edition. Knowledge Verl.-Ges, Stuttgart 2005, ISBN 978-3-8047-2113-5 , pp. 172 f .
- ↑ Reinhard Larsen: Anesthesia and intensive medicine in cardiac, thoracic and vascular surgery. (1st edition 1986) 5th edition. Springer, Berlin / Heidelberg / New York a. a. 1999, ISBN 3-540-65024-5 , p. 64 f.
- ↑ see German Migraine and Headache Society
- ↑ Instructions for use Isoptin®, Abbott 10/2006.
- ↑ Specialist information Loperamid-CT 2mg hard capsules, as of October 2008.
- ↑ U. Fuhr, H. Müller-Peltzer, R. Kern and a .: Effects of grapefruit juice and smoking on verapamil concentrations in steady state . In: Eur. J. Clin. Pharmacol. tape 58 , no. 1 , April 2002, p. 45-53 , doi : 10.1007 / s00228-002-0436-7 , PMID 11956673 . (Free full text)
- ↑ European Pharmacopoeia , Deutscher Apotheker Verlag Stuttgart, 6th edition, 2008, pp. 4298-4301, ISBN 978-3-7692-3962-1 .
- ^ Axel Kleemann , Jürgen Engel, Bernd Kutscher, Dietmar Reichert: Pharmaceutical Substances. 4th edition. 2 volumes. Thieme-Verlag, Stuttgart 2000, ISBN 1-58890-031-2 ; online since 2003 with biannual additions and updates.
- ↑ External identifiers or database links for 3,4-dimethoxyphenylacetonitrile : CAS number: 93-17-4, EC number: 202-225-1, ECHA InfoCard: 100.002.024 , GESTIS substance database : 30500 , PubChem : 66727 , ChemSpider : 60093 , Wikidata : Q27263471 .
- ↑ External identifiers or database links for N-methylhomoveratrylamine : CAS number: 3490-06-0, EC number: 222-483-9, ECHA InfoCard: 100.020.441 , PubChem : 77039 , ChemSpider : 69485 , Wikidata : Q27262167 .
- ↑ DE 1 158 083 (1963) [1] ; Dengel Ferdinand (Knoll AG); Process for the preparation of basic substituted phenylacetonitriles.
- ↑ DE 2 059 923 (1972) [2] ; Joerg Tri; Raschak Manfred, Dengel Ferdinand (Knoll AG); Optically active, basic substituted phenylacetonitrile.
- ↑ DE 2 263 527 (1973) [3] ; Yasuda Kikuo, Mori Hiromu, (Teikoku Hormone Manufg. Co., Ltd.); Alkylation phenylacetonitrile, synthesis of verapamil via reductive alkylation amines.
- ↑ EP 47 888 (1982) [4] ; Kastner Gerhard, Siegel Hardo, Geiss Karl Heinz (BASF AG); Phenylacetonitriles with basic substitutes.
- ↑ EP 165 322 (1984) [5] ; Kisielowski L. Grafe I. Liebenow W. Ahrens KH (Heumann Pharma); Process for the preparation of basic substituted phenylacetonitriles
- ↑ DE 1 154 810 (1963) [6] ; Dengel Ferdinand (Knoll AG); Basically substituted phenylacetonitriles.
- ↑ DE 3 121 766 (1982) [7] ; Seitz Werner, Scheib Klaus, Michel Alfred (BASF AG); Process for the preparation of basic substituted phenylacetonitriles.
- ↑ PL 162 512 (1993) [8] ; Chem. Abstr. 1995: 328623; Klauze-Tomaszewska Hanna, Szluker Janina. (Pabianickie Zaklady Farmaceutyczne "POLFA", Pol.); Method of isolating α-isopropyl-α - [(N-methyl-N-homoveratryl) -γ-aminopropyl] -3,4-dimethoxyphenylacetonitrile hydrochloride.
- ↑ WO 2016 181 292 [9] ; Krishnamurthy DK, Gharpure M, Somisetti NR, Rajappa M, Aareddy R, Kasireddy D .; A process for the preparation of verapamil hydrochloride.
- ↑ ROTE LISTE 2017, Verlag Rote Liste Service GmbH, Frankfurt am Main, ISBN 978-3-946057-10-9 , p. 225.
- ↑ Red List Online, as of August 2009.
- ↑ Swiss Medicines Compendium , as of August 2009.
- ↑ AGES-PharmMed, as of August 2009.

