Radiocarbon method
The radiocarbon method , also known as radiocarbon dating , 14 C ; C14-dating or radiocarbon dating or radiocarbon dating is a method for radiometric dating of carbon , in particular organic materials. The temporal range of application is between 300 and about 60,000 years.
The method is based on the fact that the proportion of bound radioactive 14 C atoms in dead organisms decreases in accordance with the law of decay . Living organisms are not affected by this effect, as they constantly absorb new carbon from the environment, which brings back the normal proportion of 14 carbon atoms. This “normal part” is almost constant in spite of the constant decay, since 14 C is constantly being newly formed in the upper atmosphere.
Radiocarbon dating was developed in 1946 by Willard Frank Libby , who was awarded the Nobel Prize in Chemistry in 1960 for this achievement . Radiocarbon dating is used in archaeological age determination , archaeobotany and Quaternary research . The limitation of the upper limit in the temporal application range results from measurement inaccuracies from ten times the half-life and external factors that have influenced the material to be examined.
Physical basics
In nature, three come isotopes of carbon before: 12 C, 13 C, 14 C . Isotope studies show that the proportion of the total carbon content in the air is around 98.89% for 12 C, around 1.11% for 13 C and around 1.25 · 10 −10 % for 14 C. Statistically speaking, there is only one 14 C nucleus for around 1 billion 12 C nuclei . In contrast to 12 C and 13 C, 14 C is not stable and is therefore also called radiocarbon.
Origin of 14 C
14 C is constantly being re-formed by nuclear reactions in the upper layers of the earth's atmosphere . When cosmic rays hit atoms in the atmosphere, spallation releases neutrons . When the most common isotope in the atmosphere, the nitrogen isotope 14 N, is hit by such a neutron, the nuclear reaction 14 N (n, p) 14 C can take place, in which this neutron is captured and a proton is split off . This creates a 14 C core from the 14 N core:
Decay of 14 C
14 C decays with a half-life of 5730 ± 40 years (so-called "Cambridge half-life") through β - decay to 14 N , one electron and one antineutrino :
During this time, the radiocarbon is widely distributed, see carbon cycle .
Equilibrium concentration
New formation and decay form a steady state. The relative equilibrium concentration of radiocarbon depends on the rate of regeneration, the half-life of decay and the amount of carbon that is in contact with the atmosphere on this time scale, where carbon is in the form of carbon dioxide . The fluctuations in this proportion are described below in the section Time fluctuations .
Start the clock by fixing it
This carbon is absorbed by plants , see carbon dioxide fixation , and stored more or less permanently or immediately introduced into the food chain . During these processes there is very little fractionation according to the isotope mass (see below), so the proportion of radiocarbon in living beings is initially almost the same as in the atmosphere. From the time of fixation, however, the proportion decreases according to the law of decay:
The ratio between 14 C and 12 C of an organic material is a measure of the age since carbon dioxide was fixed. Radiocarbon can also get into inorganic substances . One example is the carbonate in the shells of mussels. Their radiocarbon age is that of the shells plus the age of the carbon when it was built into the shell plus a slightly larger correction for fractionation. Another example is radiocarbon in alloys over charcoal used in their manufacture. The 14 C age then indicates the time of manufacture plus the age of the organic carbon used.
Radiocarbon dating is thus the measurement of the ratio of the amounts of carbon isotopes 14 C to 12 C in a sample and a standard that represents the ratio at the beginning of aging. The 14 C content of a sample can either be determined by counting the decaying 14 C nuclei in the counter tube , in the "liquid scintillation spectrometer" or by counting the 14 C nuclei still present with accelerator mass spectrometry . The latter method requires less material than the first two, but is more complex and expensive.
Procedure of the investigation
In addition to the application of physics, the implementation also requires numerous steps with the help of applied chemistry in order to be able to examine the sample with a counter tube (according to Libby), "liquid scintillation spectrometry" or with the accelerator mass spectrometry method . The following illustration of the examination process is very much simplified.
Chemical preparation of the sample
The organic material to be examined must be reduced to pure carbon in order to be able to carry out a determination. Many other substances must therefore be removed from the sample beforehand. In the following, the preparation of wood (without coniferous wood ) is shown as an example, as it is used in appropriate laboratories.
The sample is boiled overnight in 4% sodium hydroxide solution at 60 ° C. (water bath). The next day an acid-alkali-acid treatment takes place (4% hydrochloric acid 30 min, 3 times 4% sodium hydroxide solution 1 hour, 4% hydrochloric acid 1 hour). For samples that have to be measured very precisely (e.g. for calibrations), the wood is reduced to cellulose , whereby after the second leaching step the lye is replaced by sodium chlorite solution (mixed with hydrochloric acid up to pH 3).
The pure cellulose material obtained is heated up with copper (I) oxide and silver in an evacuated quartz ampoule . The organic components burn to form CO 2 , nitrogen oxide , sulfur oxide and halogen compounds . The silver binds the sulfur oxide and the halogen compounds.
The CO 2 can now be measured with a Geiger counter, or it is used for liquid scintillation in benzene converted, or it is reacted with hydrogen to iron reduced powder to graphite to then a 14 C-determining means of the accelerator mass spectrometry perform (AMS).
Libby counter tube method
The classic method for radiocarbon measurements , as already used by Libby , is the direct detection of radioactive decay in a counter tube . The CO 2 obtained from the sample by combustion is used as the counting gas . Due to the long half-life and the low abundance of 14 C, the activity of one mole of modern carbon is only about 3 decays per second. In order to achieve an accuracy of 40 years, however, a total of more than 40,000 decays must be counted. In order to achieve high measurement precision, in addition to good shielding of the counter tube against natural radiation, relatively large sample quantities (up to 1 kg of the starting material) and a long measurement period are required. Since very old samples contain very little 14 C, they can only be measured with corresponding uncertainty. With a sample age of around 50,000 years, the detection limit is considered to have been reached with an uncertainty of ± 5000 years.
Liquid scintillation spectrometry
A very common method of measuring the radioactive decay of 14 C is liquid scintillation spectrometry . The carbon to be dated is converted into benzene in a vacuum line through several intermediate stages. An organic scintillator is then added to this. The scintillator converts the energy of the electrons released during the decay of 14 C into light pulses. These are then amplified and counted in the spectrometer by highly sensitive photomultipliers . This method has the advantage over the counter tube method that more carbon can be accommodated in the measuring chamber. This enables shorter measuring times with the same accuracy. In addition, spectrometers optimized for radiocarbon dating are commercially available, while the counter tubes are in-house developments of the respective laboratories.
Accelerator mass spectrometric detection
With the development of accelerator mass spectrometry (AMS), which combines the methods of mass spectrometry and nuclear physical investigation methods and thus enables the measurement of the smallest isotope ratios down to 10 −15 , the direct detection of 14 carbon atoms became possible at the end of the 1970s without first having to wait for them to decay. Therefore, with the help of this method, much smaller sample quantities can be used than with measurements with the counter tube method, which opened up completely new areas of application for the radiocarbon method. The typical size of a sample for accelerator mass spectrometry is about 1 mg; With this amount of sample, 40,000 14 C atoms of a modern sample can be detected within a measurement time of about one hour, or a relative uncertainty of 0.5% can be achieved, which corresponds to an absolute uncertainty of 40 years. In contrast to the counter tube method , however, a far more complex and expensive technology is required for this.
Methodological boundary conditions
Detection limit
A fresh carbon sample contains only about 1 part per trillion (ppt) 14 carbon atoms. So there is one 14 C atom for every 10 12 atoms of the 12 C isotope . For example, one ton of carbon contains only 1 µg 14 C.
The detection limit of 14 C is 1 part per quadrillion (ppq), corresponding to a concentration of about one thousandth of the amount of 14 C in a fresh sample, and is limited by the limitations of the measuring devices and the "background 14 C" present in very small quantities. determined from other sources. Due to the radioactive decay , the amount of 14 C decreases over time. After 10 half-lives, that is approx. 57,300 years, the proportion is below the detection limit. The radiocarbon method can therefore only be used for younger samples. For the age determination of geological fossils z. B. in amber , lignite , hard coal or diamonds it is unusable (for example, potassium-argon dating can be used here).
Measurement accuracy
As with any physical measurement, the measurement uncertainty has a statistical and a systematic part. The statistical uncertainty is that of the isotope ratio . In the case of the counter tube method, for example, the statistical nature of the radioactive decay contributes to this. It is given by the laboratory in an easily readable form ± n years as a simple standard deviation .
In addition, the sources of error described in the next sections must be taken into account by correcting for the probable amount of the deviation and specifying their uncertainty. In particular, these are:
- All falsifications in the cleaning and preparation of the sample (rather negligible).
- All falsifications from the creation of the sample to the discovery today.
- From salts dissolved in water , dolomite precipitates in bones and is not completely dissolved at the temperatures usual for acid cleaning. These carbonates were dissolved in the water beforehand as hydrogen carbonates with carbon dioxide from the atmosphere. Very old bone samples (more than twenty thousand years old) are often measured far too young.
- All deviations in the carbon age of the sample from the age of the layer to be determined.
- All purely statistical fluctuations between apparently identical samples from the same find context.
- All deviations between the 14 C concentration of the sample material and the ambient air during its lifetime.
- In particular, marine animals and plants partially absorb carbon that comes from dissolved limestone or rising currents from the deep sea , and is measured considerably too old ( reservoir effect ).
Consideration of the find situation
The 14 C method measures the point in time at which the carbon was removed from the atmosphere or water. This is not necessarily the time when the archaeological layer was deposited. For correct dating, the connection between the termination of the carbon isotope system in the sample from the environment and the historical event to be dated must be established. For example, an oak tree can reach an age of several centuries. The 14 C examination of an object from its central heartwood does not measure the time at which the tree was felled, but rather a higher age. Here dendrochronology can provide reliable comparative values. When dating the remains of short-lived parts of plants such as plant seeds , this problem does not play a role compared to the order of magnitude of the measurement accuracy.
Libby and Cambridge half-life
Libby's team had used a half-life of 5568 ± 30 years for the development of radiocarbon dating for 14 C, after evaluating all measurement results available at the time. Later measurements revised this value to 5730 ± 40 years in 1962. Further measurements confirmed this value with further improved accuracy, so that from 1990 a decay time of 5715 ± 30 years was recommended.
Since a whole series of dating results had already been published, it was agreed to continue to use the first value ("Libby half-life") and then to designate the result as the "conventional 14 C age" because of the comparability of dating results . Compared to the use of the revised value (“Cambridge half-life”), the result is only a factor of 1.026. When it comes to determining calendar dates, this is of course taken into account, along with the other corrections that are then still required.
Variations in the atmospheric 14 C / 12 C ratio over time
Natural fluctuations
Natural temporal fluctuations in the 14 C / 12 C ratio were first demonstrated in 1958 by Hessel de Vries , who showed that the 14 C / 12 C ratio changed by about 2% between the 16th and 19th centuries. Mainly three factors play an important role for the natural fluctuations.
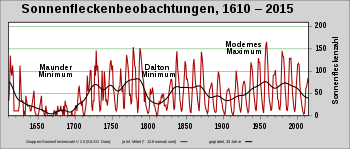
- On the one hand, there is the modulation of cosmic radiation by solar activity , which influences the production rate and causes short-term fluctuations, also known as the wiggle or DeVries effect .
- In addition, the 14 C production rate is also influenced by a change in the geomagnetic dipole field by up to a factor of three. This plays a role on time scales greater than a hundred years.
- In addition, the carbon exchange between various terrestrial carbon reservoirs and the atmosphere contributes to the fluctuation of the atmospheric 14 C / 12 C ratio.
Sometimes singular events such as near supernova explosions are also discussed.
Through measurements that led to the setting up of the INTCAL98 calibration curve , but also through some measurements that go back further in time and are based, for example, on maritime sediment drill cores from the Cariaco basin (off the north coast of Venezuela ), the deviation of the 14 C / 12 C -Ratio from today's value can be traced back up to 48,000 years. It shows that, in addition to the short-term fluctuations, a general increase in 14 C (= deviation from today's 14 C / 12 C ratio in per mille) to values up to over 800 per mille (Cariaco data) at a time around 40,000 years ago BP can be achieved, which corresponds to a difference between the radiocarbon age and calendar age of about 5000 years. Between 40,000 and 42,000 years BP, the 14 C drops abruptly to today's values and fluctuates between about 0 and −200 per thousand in the period between 42,000 years and 50,000 years BP. In addition to the peak 40,000 years ago, there are also smaller peaks 34,000 years ago, 29,000 years ago and 17,000 years ago BP. Other data sets (sediments from Lake Suigetsu , Bahamas Speleothem ) show the same structures, but show offsets for times 25,000 years ago compared to the data from the Cariaco drill core , which, when converted into radiocarbon years, are roughly in the order of magnitude 1000 years ago.
A comparison of this data with calculated production rates, which included published changes in the geomagnetic field over the corresponding period, shows that the long-term changes and the peaks found can generally be explained well by changes in the earth's magnetic field, with reduced carbon sinks during periods of icing and others Changes in the carbon cycle appear to play a role. The peaks 34,000 years ago and 40,000 years ago coincide well with the peaks of the radionuclides 36 Cl and 10 Be , which were detected in ice cores . For short-term fluctuations, a correlation with solar activity and the temperature of the northern hemisphere could be demonstrated. The 14 C production rates are lower when solar activity ( sunspots ) is high.
Sweet effect
The Suess effect is named after Hans E. Suess (1909–1993) and describes the influence of industrialization on the 14 C content in the atmosphere. With the beginning of industrialization around 150 years ago, fossil fuels such as crude oil and coal were increasingly used. These substances no longer contain any detectable 14 C, as they are much older than approx. 10 half-lives (approx. 60,000 years). This can simulate that the examined sample is too old, because when fossil fuels are burned , only 12 C and 13 C (non-radioactive) are released and dilute the amount of radioactive 14 C in the atmosphere. The dilution of the 14 C in the atmosphere leads to a reduced initial value of the 14 C in the organisms, which must be taken into account when determining the 14 C age.
Nuclear effect
The use and atmospheric tests of nuclear weapons between 1945 and 1963 greatly increased the amount of 14 C in the atmosphere. To date, the 14 C / 12 C ratio has not fallen back to the value it had before 1945. There are reference samples for each year, and because of the large variation, samples can be dated to within ± 1 year.
The massive local generation of 14 C in the atmosphere caused by the nuclear weapons tests could be used to precisely investigate the temporal behavior and, above all, the spatial transport process of 14 C. This confirmed that 14 C in the atmosphere was homogenized worldwide within a short period of time . Thus, an earlier research result by Ernest C. Anderson on the spatial homogeneity of 14 C in the atmosphere was confirmed. This homogeneity is an important prerequisite for the calibration and application of the 14 C method.
Corrections to the measurement
Fractionation
Since the isotopes 12 C, 13 C and 14 C have different weights, they are moved or released slightly differently during transport processes and chemical reactions ( isotope fractionation ), so that their mixing ratio changes. In photosynthesis z. B. This reduces the ratio of 14 C to 12 C in the plant compared to the air. The same applies to the ratio of 13 C to 12 C, although the effect here is only half as great. Due to the high proportion of 13 C in the total carbon of approx. 1%, this effect can be measured relatively easily at 13 C, so that the effect for 14 C can then be calculated and taken into account when determining the age.
An important difference in isotope fractionation exists, for example, between C3 plants and C4 plants . The 13 C / 12 C ratio can also provide important information about nutrition based on bones, for example.
Reservoir effect and hard water effect
In certain cases the starting concentration of the object to be dated is not that of the atmosphere, but that of another larger carbon reservoir. The dissolved carbonic acid used to date groundwater, for example, is composed of carbonate from the subsoil, which was formed a long time ago, i.e. is 14 C-free, and the carbon dioxide formed by the decomposition of plant material in the soil. If this is not included in the age calculation, the age is determined to be too great (hard water error). Fish freshly caught in Antarctica would also be 14 C-dated several hundred years ago if one did not take into account that they take up carbon from water that forms from very old ice masses via the food chain.
contamination
A further correction may be necessary if the measured sample has been contaminated by a substance with a different radiocarbon age and this contamination could not be completely removed by the cleaning procedures during sample preparation. Depending on the extent of the contamination, the measured radiocarbon age is between the age of the sample and the radiocarbon age of the contaminating substance . If the extent of the contamination is known, the following formula applies to the shift from the measured radiocarbon age to the real age of the sample:
- are the contamination in%, the radiocarbon age of the contamination or the age of the sample.
If contamination is suspected but the exact extent is not known, a sample can be divided into several sub-samples and different chemical cleaning procedures can be carried out on each sub-sample. As a rule, this means that the actual sample material and any contamination that may be present are attacked to different degrees and the ratio of the two changes differently in the individual sub-samples. A contamination that noticeably falsifies the age is then noticeable in the strongly diverging dates of the partial samples. This can serve as a criterion for inferring the reliability of a radiocarbon age.
calibration
The raw date obtained as a measurement result from a conventional 14 C or AMS laboratory with the associated standard deviation always relates to the year 1950, but this does not include a calendar value because it is based on a constant 14 C formation rate that does not correspond to reality . At the end of the 1950s, scientists discovered that the production of 14 C isotopes and thus the 14 C content of the atmosphere was and is subject to considerable fluctuations over the course of the Earth's history , which is due to short and long-term cycles of solar activity and fluctuations in the Earth's magnetic field caused. The misnomer "BP", which is common for these raw data referring to 1950, can lead to misunderstandings because of the obvious translation "before today".
Because for the reasons presented, 14 C raw data can lead to deviations of up to several thousand solar years and must therefore be corrected. This conversion of the 14 C raw data is referred to as calibration and is specified in the English-language literature as calBP . For further conversion into our general time calculation for scientifically unambiguous comparisons, especially in archeology and prehistory , the 1950 years must be subtracted from the calibrated information and added cal. v. Chr. Are clearly identified (English "calBC" for before Christ or "BCE" for before the Common Era ) are used. Each of these items of information includes the variation information and its size (1 or 2 σ), which is often already included in the calculation, with the older and younger limit being indicated, e.g. B. "5555-5247 cal. v. Chr. "
The current status was published in 2013 and incorporated into the English "OxCal" program. Version 4.4 will be available in 2020.
The Cologne program "CALPAL" was exemplary, clear and easy to use, but apparently remained unchanged from 2007.
Another program is available from the University of Washington's Quaternary Isotope Lab .
The calibration of individual archaeological data> 26,000 BP up to around 50,000 BP is still controversial, since calibration curves only give an averaged value of the deviation from solar years, which can be much higher in individual cases. In the case of the INTCAL13 curve, only the time range up to 12,594 cal BP, secured by means of dendrochronology, is regarded as a high-resolution range in calendar years .
Calibration by dendro curves
In the early 1960s, the first calibration curves were created based on the dendrochronology of particularly long-lived trees such as the bristlecone pine and the giant sequoia . In the meantime the system of dendrochronology could be extended to many parts of the world. The Bristlecone-Pines chronology now goes back over 9,000 years. The Hohenheim annual ring calendar extends completely back to the year 10 461 BC. Back to the Younger Dryas (2004). The Eastern Mediterranean curve covers the period up to 1800 BC. Chr.
- Calibration curve

The laboratory data initially gives the (“conventional”) 14 C age B.P. (before = before, present = standardized to 1950) including the associated standard uncertainty. From this, with the aid of the calibration procedure described, the calibrated calendar age in cal B.P. can be determined with further information on the spread. BC (BC) or AD (AD = Anno Domini ).
If the calibration curve is flat over a longer section (one then speaks of a plateau), this means that bones or charcoal , the origin of which are several hundred years apart, have the same conventional 14 C age. This is the case, for example, with the ceramic band plateau between 5500 and 5200 BC cal, then again in the areas 3100-2900, 2850-2650 and 2600-2480 BC ( end neolithic plateau).
In the meantime, the fluctuations in the calibration curve are also used to specify 14 C dates, e.g. B. through the "wiggle matching" developed by Bernhard Weninger from the University of Cologne . This is possible if precise data is available, the relative sequence of which has been proven by independent sources, such as the stratigraphy of an archaeological site. This allows a decision to be made as to which section of the calibration curve these data best fit into.
Calibration with varves
Calibration using varven chronology (band tone dating) is increasingly playing a role, as this allows the high-resolution range of annual growth events to be expanded far beyond the archives of tree ring data. The varven chronology published in 2012 from Lake Suigetsu, Japan, dates back 53,000 years, making it the world's longest known archive of warmed lake sediments to date . The significantly improved calibration of data older than 11,200 14 C-years was first published in the same issue of Science .
Research history
The possibility of dating by measuring 14 C was shown for the first time in 1949 by the "Curve of Knowns" published by J. R. Arnold and W. F. Libby, in which the age of known samples shows the inverse dependence of the 14 C content on the age of each Sample was shown. Until then, the main focus was on metrological problems, in particular the differentiation of the relatively weak signal from the radioactive decay of 14 C from the background signal of ambient radioactivity .
In the following years, some requirements for reliable dating by means of radiocarbon dating were checked. The assumption could be confirmed that the 14 C / 12 C ratio in the global atmosphere is spatially sufficiently homogeneous or, in the worst case, leads to local deviations which are in the order of magnitude of the other measurement accuracy of radiocarbon dating. At the latest with the work of Suess and deVries, however, it became clear that the 14 C / 12 C ratio is subject to fluctuations over time, which must be taken into account for accurate dating by radiocarbon dating.
This discovery led to the development of calibration curves from the beginning of the 1960s, which were initially based on dendrochronologies from long-lived giant sequoia trees and bristlecone pines . Subsequent precision calibration curves were established with the help of dendrochronologies from shorter-lived trees such as the Hohenheim tree- ring calendar . In addition to dendrochronology, other independent methods (measurements on corals , ice cores, sediment layers , stalagmites ) were later increasingly used to check and extend the calibration curves based on dendrochronology. This led to the INTCAL04 calibration curve published in 2004, which goes back to 26,000 BP.
Another milestone was the use of accelerator mass spectrometry (AMS) for radiocarbon dating by Harry Gove in 1977. This enabled radiocarbon dating to be carried out on much smaller sample quantities than with the counter tube method.
In August 2020, updated calibration curves for the mainland and the oceans were published in the journal Radiocarbon .
Trivia
The radiocarbon method is also used to measure transport routes and transport mechanisms of plant constituents. To do this, the plants are fumigated with CO 2 , which was obtained from crude oil, natural gas or natural gas wells and therefore no longer contains any detectable amounts of the 14 C isotope . The isotope ratio then serves as an indicator, similar to a tracer . In the course of photosynthesis the plants thus build ingredients, which are examined by scintillation spectrometry in the various parts of the plant (so that can lower tracks than when measured by CO 2 - sensors are detected). For example, it was used to determine plant exudates and their effects on root respiration and soil respiration .
literature
Books
- Hans Mommsen : Archaeometry . Newer scientific methods and successes in archeology. Teubner, Stuttgart 1986, ISBN 3-519-02654-6 .
- Roman Laussermayer: Meta-physics of the radiocarbon dating of the Turin shroud . Critical analysis and new interpretation of the dating results. VWF, Berlin 2000, ISBN 3-89700-263-9 .
- A. J. Shortland & C. Bronk Ramsey (Eds.): Radiocarbon and the Chronologies of Ancient Egypt. Oxbow Books, Oxford, UK, 2013, ISBN 978-1-84217-522-4 , ( table of contents ).
- Mebus A. Geyh: The application of the 14C method. Clausthaler Tektonische Hefte, 11, EPV Clausthal-Zellerfeld 1971 ( pdf 6 MB ).
- R. Wauchope: Implications of radiocarbon dates, From Middle and South America. Tulane University, New Orleans 1954.
Essays
- Lloyd A. Currie: The remarkable metrological history of radiocarbon dating [II]. In: Journal of Research of the National Institute of Standards and Technology. 109, 3, 2004, doi: 10.6028 / jres.109.013 .
- Michael Friedrich, Sabine Remmele, Bernd Kromer, Jutta Hofmann, Marco Spurk, Klaus Felix Kaiser, Christian Orcel, Manfred Küppers: The 12,460-Year Hohenheim Oak and Pine Tree-Ring Chronology from Central Europe. A Unique Annual Record for Radiocarbon Calibration and Paleoenvironment Reconstructions. In: Radiocarbon . 46, 3, 2004, pp. 1111-1122, online .
- Olaf Höckmann : On the problem of the application of scientific dating methods in archeology. In: Hans-Günter Buchholz (Ed.): Aegean Bronze Age. Wissenschaftliche Buchgesellschaft, Darmstadt 1987, ISBN 3-534-07028-3 , pp. 29-52.
- Paul Mellars: A new radiocarbon revolution and the dispersal of modern humans in Eurasia. In: Nature . 439, 2006, doi: 10.1038 / nature04521 .
- Gerhard Morgenroth: Radiocarbon dating. Xerxes' false daughter. In: Physics in Our Time . 34, 1, 2003, doi: 10.1002 / piuz.200390008 .
- Minze Stuiver, Henry A. Polach: Discussion: Reporting of 14 C Data. In: Radiocarbon. 19, 3, 1977, pp. 355-363, digitized version (PDF; 125 kB) ( Memento from July 16, 2007 in the Internet Archive ).
Individual evidence
- ^ Willard F. Libby : Radiocarbon Dating. University of Chicago Press, Chicago 1952, ISBN 978-0-226-47980-4 .
- ↑ Harry Godwin : Half-life of radiocarbon. In: Nature . 195, 1962, p. 984, doi: 10.1038 / 195984a0 .
- ↑ N. E. Holden: Total half-lives for selected nuclides. In: Pure and Applied Chemistry. 62, 5, 1990, doi: 10.1351 / pac199062050941 .
- ↑ St. De Vries: Variation in concentration of radiocarbon with time and location on earth. In: Proc. Royal Nederl. Akad. Wetenschappen. B, 61, 2, 1958, pp. 94-102.
- ↑ a b M. Stuiver et al .: INTCAL98 Radiocarbon Age Calibration, 24000-0 cal BP. In: Radiocarbon. 40, 3, 1998, pp. 1041-1083, online .
- ^ A b K. Hughen, S. Lehman, J. Southon, J. Overpeck, O. Marchal, C. Herring, J. Turnbull: 14C Activity and Global Carbon Cycle Changes over the Past 50,000 Years. In: Science . 303, 2004, pp. 202–207, ( free full text ( Memento from January 20, 2012 in the Internet Archive )).
- ↑ P. E. Damon, J. C. Lerman, A. Long: Temporal Fluctuations of Atmospheric 14C, Causal Factors and Implications. In: Annual Review of Earth and Planetary Sciences. 6, 1978, pp. 457-494, ( free full text ).
-
↑ Data from Trends: A Compendium of Data on Global Change. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge, Tenn., USA
- M. R. Manning, and W. H. Melhuish. 1994. Atmospheric δ14C record from Wellington ( Memento from February 1, 2014 in the Internet Archive ) (English)
- I. Levin, B. Kromer, H. Schoch-Fischer, M. Bruns, M. Münnich, D. Berdau, J. C. Vogel, and K. O. Münnich, 1994. δ14CO2 record from Vermunt (English)
- ^ A b E. C. Anderson, W. F. Libby: Worldwide distribution of natural radiocarbon. In: The Physical Review. 81, 64, 1951, pp. 64-69, doi: 10.1103 / PhysRev.81.64 .
- ^ A b H. E. Suess: Radiocarbon concentration in modern wood. In: Science. 122, 1955, doi: 10.1126 / science.122.3166.415-a .
- ↑ a b St. De Vries: Variation in concentration of radiocarbon with time and location on earth. In: Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen. B, 61, 2, 1958, pp. 94-102.
- ↑ Bernhard Weninger, Uwe Danzeglocke, Olaf Jöris: Comparison of Dating Results achieved using Different Radiocarbon-Age Calibration Curves and Data. ( PDF download , source www.calpal.de ).
- ↑ Paula J. Reimer, Edouard Bard, Alex Bayliss et al .: Intcal13 and Marine13 radiocarbon age calibration curves 0-50,000 years cal BP. In: Radiocarbon. 55, 4, 2013, doi: 10.2458 / azu_js_rc.55.16947 (free full text).
- ^ Bernhard Weninger, Olaf Jöris: Glacial radiocarbon age calibration: the CalPal program. In: Thomas Higham et al. (Ed.): Radiocarbon and Archeology: Fourth international symposium. Oxford University School of Archeology, Oxford 2004, ISBN 978-0-947816-65-0 , pp. 9-15.
- ↑ U. Danzeglocke, O. Jöris, B. Weninger: CalPal-2007online (website CalPal)
- ↑ the CALIB program's website ( Memento of 13 August 2011 at the Internet Archive )
- ^ Paula J. Reimer: Refining the Radiocarbon Time Scale. In: Science. 338, 2012, doi: 10.1126 / science.1228653 ( free full text ).
-
↑ Christopher Bronk Ramsey et al .: A Complete Terrestrial Radiocarbon Record for 11.2 to 52.8 kyr B. P. In: Science. 338, 2012, doi: 10.1126 / science.1226660 ( free full text ).
'Time-capsule' Japanese lake sediment advances radiocarbon dating for older objects. - ^ J. R. Arnold, W. F. Libby: Age Determinations by Radiocarbon Content, Checks with Samples of Known Age. In: Science. 110, 1949, doi: 10.1126 / science.110.2869.678 ( online ( Memento from July 22, 2014 in the Internet Archive )).
- ↑ E. K. Ralph, H. N. Michael: Twenty-five years of radiocarbon dating: The long-lived bristlecone pines are being used to correct radiocarbon dates. In: American Scientist . 62, 5, 1974, pp. 553-560.
- ↑ P. J. Reimer (Ed.): IntCal04: Calibration Issue. In: Radiocarbon. 46, 3, 2004, ( special issue, Open Access ).
- ↑ Harry E. Gove: From Hiroshima to the Iceman. The Development and Applications of Accelerator Mass Spectrometry. Institute of Physics Publishing, Bristol 1999, ISBN 0-7503-0557-6 .
- ↑ C14 method: research team re-calibrates radiocarbon watch for age determination. On: idw-online.de from August 17, 2020. See: doi: 10.1017 / RDC.2020.41 , doi: 10.1017 / RDC.2020.59 and doi: 10.1017 / RDC.2020.68 and doi: 10.1017 / RDC.2020.46 .
Web links
- Methodological explanation of the University of Kiel
- Chemical pre-treatment
- Overview of the calibration programs (online and as download)
- [1] (OxCal, link June 2021 adjusted)
- CalPal calibration program
- Comparison of different calibration curves and programs ( Memento from July 18, 2011 in the Internet Archive ) (PDF; 82 kB)
- Radiocarbon magazine
- List of all active radiocarbon dating laboratories
- RADON - database for European 14 C data