Uraninite
| Uraninite | |
|---|---|
| Uraninite from the Chestnut Flats Mine near Spruce Pine, Mitchell County, North Carolina, USA (size: 4 cm × 2.4 cm × 2.1 cm) | |
| General and classification | |
| other names |
|
| chemical formula | UO 2 |
|
Mineral class (and possibly department) |
Oxides and hydroxides - oxides with metal: oxygen = 1: 2 |
|
System no. to Strunz and to Dana |
4.DL.05 ( 8th edition : IV / D.31) 05.01.01.01 |
| Similar minerals | Thorianite , coffinite |
| Crystallographic Data | |
| Crystal system | cubic |
| Crystal class ; symbol | cubic hexakisoctahedral; 4 / m 3 2 / m |
| Space group | Fm 3 m (No. 225) |
| Lattice parameters | a = 5.47 Å |
| Formula units | Z = 4 |
| Physical Properties | |
| Mohs hardness | 5 to 6 |
| Density (g / cm 3 ) | measured: 10.63 to 10.95; calculated: 10.88 |
| Cleavage | Well |
| Break ; Tenacity | shell-like to uneven, brittle |
| colour | gray, black, brownish |
| Line color | brown-black to greenish |
| transparency | opaque (splinters and thin layers translucent) |
| shine | Greasy to metallic sheen, matt |
| radioactivity | very radioactive |
| Other properties | |
| Special features | often brightly colored oxidation products |
Uraninite , also known as pitchblende or beechblende , is a common mineral from the mineral class of " oxides and hydroxides ". It crystallizes in the cubic crystal system with the composition UO 2 , so it is chemically a uranium (IV) oxide . Due to the radioactive decay of uranium , uraninite always contains a certain amount of lead oxide (PbO), which can be up to 20% depending on the geological age.
Uraninite usually develops cube-shaped or octahedral crystals or their combinations, but also kidney, granular or massive aggregates in gray, black and brownish color with brownish-black to greenish streak color . In general, uraninite is opaque, only fine splinters and thinnest layers are translucent red-brown. Fresh samples have a pitch-like to greasy, occasionally slightly metallic sheen , which, however, becomes matt after some time due to weathering.
Etymology and history
One of the oldest mentions of the mineral was made in 1565 by Johannes Kentmann , who called it Plumbago sterilis pici similis Bechblende (pitch-like sterile diaphragm ). He took over this from the Saxon miners who extracted the mineral from the silver - cobalt passages of the Ore Mountains . These had no use for the pitch black stones and discarded the supposedly metal-free cover .
When different colored oxidation products were later to be found on the discarded pitchblende in the old heaps, they were dismantled to obtain these beautiful new colors. When the already oxidized materials were used up, the paints were also made from pitchblende to a certain extent. Therefore, today some old works of art are radioactive. Since it was recognized that the pitchblende consists of a compound of different uranium oxides that are deposited as a collomorphic aggregate , the term has only been used for this mixture.
Martin Heinrich Klaproth was 1789 from pitchblende the element uranium isolate, which he initially as Uranit called, in 1790, however, the rules of analogy according to Uranium renamed. The term uranite was subsequently used as a synonym for various uranium minerals. Klaproth also gave the erroneous name sulphurized uranite . Later he took over the name uranium ore coined by Karsten in 1792 . Other synonyms are Pecherz (from Werner ), Uranpecherz (from Leonhard ), Pechuran (from Hausmann ) and Nasturan (from Kobell 1853 from Greek ναστός nastós for dense or coarse). Haidinger finally introduced the term uranine as a name for the mineral in 1845 , which was transferred in 1868 by James Dwight Dana to the name uraninite (e), which is still valid today.
Although the mineral was known earlier as described, the type locality for uraninite is the St. Joachimsthal vein deposit (today Jáchymov ), from where Franz Ernst Brückmann described it in 1727. The pitchblende that Klaproth used to discover uranium comes from the Georg Wagsfort mine in Johanngeorgenstadt in the Saxon Ore Mountains . In contrast, the French physicist Henri Becquerel did not use pitchblende, as is often claimed, to discover radioactivity in 1896, but rather artificially produced uranium compounds. The Polish-French chemist and Nobel Prize winner Marie Curie used for their research leading to the discovery of uranium - decay products polonium and radium led, initially pitchblende. For cost reasons, however, it mainly used the tailings from the uranium dye production in Jáchymov, in which these rare elements were already enriched compared to the original ore. One ton contains about 0.1 g of radium.
classification
In the meanwhile outdated, but still in use 8th edition of the mineral classification according to Strunz , the uraninite belonged to the mineral class of "oxides and hydroxides" and there to the department of "oxides with the molar ratio of metal: oxygen = 1: 2", where it was used together with baddeleyite , Calcirtite , cerianite (Ce) , hiärneit , tazheranite and thorianite formed a separate group.
The 9th edition of Strunz's mineral systematics , which has been in effect since 2001 and is used by the International Mineralogical Association (IMA), also assigns uraninite to the class of "oxides and hydroxides" and there in the department of "metal: oxygen = 1: 2 and comparable" a. However, this section is further subdivided according to the size of the cations involved and the crystal structure, so that the mineral according to its composition and structure is classified in the sub-section “With large (± medium-sized) cations; Structures typical of fluorite ”can be found where it forms the unnamed group 4.DL.05 together with cerianite (Ce), thorianite and zircelite .
The systematics of minerals according to Dana , which is mainly used in the English-speaking world , assigns uraninite to the class of "oxides and hydroxides", but there it belongs to the category of "uranium and thorium-containing oxides". Here it can only be found together with thorianite in the unnamed group 05.01.01 within the subdivision of " Oxides containing uranium and thorium with a cation charge of 4+ (AO 2 ) ".
Crystal structure
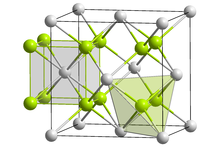
Uraninite crystallizes in the cubic crystal system in the space group Fm 3 m (space group no. 225) with the lattice parameter a = 5.47 Å and four formula units per unit cell . Its crystal structure is isotype with fluorite (CaF 2 ). The U 4+ - cations thereby form a cubic close packing , the tetrahedral holes completely from oxide - anions are occupied, that is, each oxygen atom is four uranium atoms tetrahedrally surrounded. The uranium (IV) cations in turn have a coordination number of CN = 8, resulting in a cube as a coordination polyhedron .
properties
With its uranium content of up to 88.15%, uraninite is one of the strongest natural sources of ionizing radiation . If the uranium is in secular equilibrium with its daughter nuclides , pure uraninite has a specific activity of around 157.8 kBq / g (for comparison: natural potassium 0.0312 kBq / g; spent nuclear fuel 18,000,000 kBq / g). Therefore, uraninite should only be stored and processed under appropriate safety precautions.
Uraninite is usually at least partially metamictic (isotropic), i. H. its crystal lattice was partially or largely destroyed by its own radioactivity , but in contrast to other, less uranium-poor minerals, remnants of the crystal lattice are mostly still detectable. Nevertheless, certain physical properties such as cleavage are lost and the samples, which still appear crystalline on the outside, show a shell-like break.
Also of interest is the high variability of the density , which is initially around 10.63 to 10.95 g / cm³, but slowly sinks to around 9 to 7.5 g / cm³ with increasing geological age. Coarse and collomorphic varieties can become comparatively light, especially when weathered, and even fall well below 7. Uraninite is often accompanied by brightly colored (red, yellow, rarely green) weathering products.
Mineral rarely columbite epitaxies form. Uraninite crystals grow on columbite crystals in certain directions. It forms the first end link of the perfect mixture series ( mixed crystal ) uraninite- thorianite . Thorium containing uranium Destinite are among other Bröggerit called. Younger uraninites have a glass to pitch-like shine, while the older specimens have a more and more metallic sheen. Weathering influences and metamorphosis make the metallic luster disappear again.
Modifications and varieties
Pitchblende essentially consists of U 3 O 8 , more rarely also U 3 O 7 plus other metal oxides with lead, iron, thorium and rare earth metals and was given the name because of its often black color and greasy sheen, similar to that of the tar-like substance pitch looks very similar.
Nierig-spherical varieties are called bladder ore. Greasy, shiny, coarse variants are called pecherz. When individual balls protrude from the matrix, they are often called mouse eyes because of their black color. Thorium-containing pegmatically formed pieces are called bröggerite. Rich ore only refers to pieces that contain a relatively large amount of a sought-after mineral. The name isn't just limited to uraninite.
Education and Locations
Uraninite / pitchblende occurs in the following deposits:
- hydrothermal corridors (Saxon-Bohemian Ore Mountains ; Příbram , Czech Republic; Krunkelbachtal near Menzenschwand , Black Forest ; Massif Central , France)
- Discordance-bound deposits ( Athabasca Basin, Canada; Northern Territory , Australia)
- Sediment-bound deposits in sandstones, carbonates, coal (Königstein, Saxony; Culmitzsch, Thuringia ; Freital , Saxony ; Curnamona Province, South Australia; Colorado Plateau, USA; Niger)
- Black slate-bound deposits ( Ronneburg , Thuringia)
- Iron-Oxide-Copper-Gold deposits ( Olympic Dam , South Australia)
- bound to volcanic rocks (Streltsovka Caldera near Krasnokamensk, Russia; Delitzsch , Saxony)
- Pegmatite (Norway)
use
Uraninite is the economically most important uranium mineral . In the 19th and early 20th centuries, uraninite was extracted for the production of paints and for the extraction of radium (e.g. Jáchymov (Joachimsthal) , Czech Republic). During the Cold War there was a need for uranium for the manufacture of nuclear weapons and for the manufacture of plutonium in nuclear reactors that went well beyond energy production . In Ore ranging there uranium deposits were the S owjetisch- D eutsche A ktien g SOCIETY (SDAG) bismuth in the DDR degraded in large scale and in pre-processed form ( Seelingstädt , Crossen ) in the Soviet Union accommodated. From the 1970s, the production of uranium for energy production dominated.
With the political change from 1989 on, there was a sharp drop in uranium production. At that time, uranium production for nuclear weapons by the two superpowers no longer played an essential role, but both sides had amassed large strategic reserves, which were released and severely depressed the world market price. In addition, new producers with low prices in Central Asia now appeared on the free market.
Precautions
Due to the toxicity and radioactivity of the mineral, mineral samples should only be kept in dust- and radiation-tight containers, but especially never in living rooms, bedrooms or workrooms. Absorption into the body ( incorporation or ingestion ) should also be prevented in any case and, for safety, direct body contact should be avoided and respiratory protection mask and gloves should be worn when handling the mineral .
See also
literature
- Paul Ramdohr , Hugo Strunz : Klockmann's textbook of mineralogy . 16th edition. Ferdinand Enke Verlag, Stuttgart 1978, ISBN 3-432-82986-8 , p. 545-548 .
- Petr Korbel, Milan Novák: Encyclopedia of Minerals . Nebel Verlag GmbH, Eggolsheim 2002, ISBN 3-89555-076-0 , p. 108 (uranium pecherz) .
- Hans Lüschen: The names of the stones. The mineral kingdom in the mirror of language . Ott Verlag, Thun and Munich 1968, p. 288 .
Web links
- Mineral Atlas: Uraninite (Wiki)
- Thomas Witzke : The discovery of uraninite, pitchblende
- mindat.org - Uraninite (English)
- Mineralogy Database - uranium Destinite (English)
- RRUFF Database-of-Raman-spectroscopy - Uraninite (English)
Individual evidence
- ^ LJ Spencer: A (Sixth) List of New Mineral Names . In: Mineralogical Magazine . tape 16 , no. 77 , 1913, pp. 374 (English, rruff.info [PDF; 1,2 MB ; accessed on January 7, 2019]).
- ↑ a b c p Greaux, L. Gautron, D. Andrault, N. Bolfan-Casanova, N. Guignot, J. Haines: Structural characterization of natural UO 2 at Pressures up to 82 GPa and Temperatures up to 2200 K . In: American Mineralogist . tape 93 , no. 7 , 2008, p. 1090-1098 ( abstract [PDF; 396 kB ; accessed on March 26, 2018] Title overview of the volume with link to the full text for members ).
- ↑ Uraninites . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 ( handbookofmineralogy.org [PDF; 110 kB ]).
- ↑ a b c Webmineral - Uraninite (English)
- ↑ TG Kotzer, TK Kyser: O, U, and Pb isotopic and chemical variations in uraninite: Implications for deterministic mining the temporal and fluid history of ancient terrains . In: American Mineralogist . tape 78 , no. 11-12 , 1993, pp. 1262–1274 ( minsocam.org [PDF; 1.7 MB ; accessed on March 26, 2018]).
- ↑ a b Friedrich Klockmann : Klockmanns textbook of mineralogy . Ed .: Paul Ramdohr , Hugo Strunz . 16th edition. Enke, Stuttgart 1978, ISBN 3-432-82986-8 , pp. 545 (first edition: 1891).
- ↑ Filippo Bianconi: Two hundred years of uranium: A historical overview . In: Association of Friends of Mining in Graubünden - Communications 52 . tape 2 , May 1990, pp. 15 ( bergbau-gr.ch [PDF; 2.0 MB ; accessed on March 26, 2018]).
- ^ Willhelm Haidinger: Handbook of determining mineralogy . Braumüller & Seidel, Vienna 1845, p. 546–555 ( rruff.info [PDF; 656 kB ; accessed on March 26, 2018] Second class: Geogenide. XI. Okay, ores. VII. Uranium ore. Uranine; P. 549).
- ↑ James Dwight Dana, George Jarvis Brush: A System of Mineralogy: Descriptive Mineralogy, Comprising the Most Recent Discoveries . 5th edition. Wiley & Son, New York 1868, pp. 154 ( limited preview in Google Book search).
- ↑ F. Veselovsky, P. Ondrus, A. Gabsová, J. Hlousek, P. Vlasimsky, IV Chernyshew: Who was who in Jáchymov mineralogy. T. 2. In: Journal of the Czech Geological Society. Prague 48.2003, 3-4, 193-205. ISSN 0008-7378
- ↑ IAEA: Facilitating Radioactive Waste Management Co-operation with the Russian Federation (English)
- ↑ Martin Okrusch, Siegfried Matthes: Mineralogie: An introduction to special mineralogy, petrology and deposit science . 7th edition. Springer Verlag, Berlin, Heidelberg, New York 2005, ISBN 3-540-23812-3 , pp. 57 .