Benzoic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
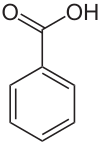
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Benzoic Acid ( IUPAC , Common) | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 7 H 6 O 2 | |||||||||||||||||||||
| Brief description |
colorless solid with a characteristic odor |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 122.12 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.27 g cm −3 (20 ° C) |
|||||||||||||||||||||
| Melting point |
122 ° C |
|||||||||||||||||||||
| boiling point |
250 ° C |
|||||||||||||||||||||
| Vapor pressure |
0.001 h Pa (20 ° C) |
|||||||||||||||||||||
| pK s value |
4.2 |
|||||||||||||||||||||
| solubility | ||||||||||||||||||||||
| Refractive index |
1.504 (132 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data |
|
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Benzoic acid [ ˈbɛnt͜soeˌzɔɪ̯ʀə ] is an aromatic carboxylic acid . It consists of a phenyl radical with a carboxy group . The salts and esters of benzoic acid are called benzoates .
etymology
Benzoic acid was named after its starting material, benzoin . This name is of Malaysian origin and was originally called "lubân djawi" = incense from Java . With the drug of the same name, the name migrated west, initially to the Mediterranean region. The elimination of the initial lu- became via banjawi , beijoim , belzui , and finally benzoe .
Occurrence
As a main component of the resin benzoin , benzoic acid is contained in frankincense - mainly in the Russian Orthodox area. Benzoin is the resin of two tree species from the group of Storax trees ( Styracaceae ), the " Siam Benzoin " ( Styrax tonkinensis ) and the " Sumatran Benzoin " ( Styrax benzoin ), both of which are native to Southeast Asia. Benzoic acid is also found in fruits, for example in the paradise apple Malus pumila , cranberries (up to 0.24% content), raspberries , blueberries and plums (content 0.1–0.2%) and in the defense secretion of various swimming beetles of the genus Dytiscus . Benzoic acid is also found in many foods such as milk and dairy products as well as honey .
properties
Benzoic acid forms colorless, shiny flakes or needle-shaped crystals, which are only slightly soluble in cold water and more readily soluble in warm water. At over 370 ° C, benzoic acid slowly decomposes into benzene and carbon dioxide (CO 2 ). Benzoic acid has an intense odor and is easily combustible. The flash point is 140 ° C, the ignition temperature is 570 ° C
presentation
Benzoic acid can be prepared from bromobenzene using a Grignard reaction . The bromobenzene reacts with magnesium to form phenylmagnesium bromide , which reacts with carbon dioxide to form C 6 H 5 COOMgBr and finally to benzoic acid by adding a hydrochloric acid solution.
Further, benzoic acid can be prepared by carboxylation of benzene by means of phosgene and aluminum trichloride (AlCl 3 ) can be represented as a catalyst. This reaction consists of a Friedel-Crafts acylation and subsequent hydrolysis of the intermediate benzoyl chloride :
Technically, toluene is oxidized with manganese dioxide and sulfuric acid in the presence of manganese naphthenate , or by reaction with potassium permanganate . Today, toluene is industrially oxidized with oxygen in the gas phase in the presence of catalysts such as vanadium pentoxide .
use
Benzoic acid is used in the production of benzoic acid esters , which are used in the perfume industry as fragrances (such as ethyl benzoate ) or as biocides ( e.g. benzyl benzoate ). Benzoic acid is also used for plasticizers in the preparation of benzoyl compounds such as benzoyl chloride and dibenzoyl peroxide .
In the food industry, benzoic acid (E 210) is often used as a preservative in long- life foods , such as ketchup, mustard and other sauces, as well as sausage, margarine, fish salads and many other products, but especially for pickled foods. Salts are more common because of their better solubility: Sodium benzoate (E 211), potassium benzoate (E 212), calcium benzoate (E 213). Benzoic acid is also approved as a feed additive for fattening pigs in the European Union .
Benzoic acid is also often used as a preservative in tobacco products. The Tobacco Ordinance permits the use of benzoic acid and sodium benzoate.
Benzoic acid is used to treat skin fungi and is approved for the preservation of cosmetics in accordance with the German Cosmetics Ordinance . The bacteriostatic and fungistatic effect is based on the inhibitory effect on enzymes that break down reactive oxygen species ( catalase and peroxidase ), which creates an accumulation of hydrogen peroxide in the cells of the microorganisms. This ultimately leads to their death.
In environmental monitoring , floor traps are filled with saturated benzoic acid solution in order to kill captured organisms such as insects or snails and to hold them in place until the next emptying.
Benzoic acid is a primary substance according to the Pharmacopoeia .
toxicology
Benzoic acid is a substance that damages the lungs and can cause asthma symptoms. It is corrosive and can cause serious eye damage. It is also discussed in connection with concentration problems and hyperactivity in children. The BfR pointed to the possible formation of small amounts of toxic benzene from benzoic acid in combination with ascorbic acid (vitamin C) in soft drinks and fruit juices.
Web links
- Extensive information from the consumer initiative on the subject of benzoic acid
- The Chemically Preserved Environment - Radiofeuilleton Meal
Individual evidence
- ↑ entry to benzoic acid in the CosIng database of the European Commission, accessed on 14 January 2020th
- ↑ Entry on E 210: Benzoic acid in the European database for food additives, accessed on August 15, 2020.
- ↑ a b c d e f g h i j k l Entry on benzoic acid in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Benzoic acid data sheet (PDF) from Merck , accessed December 28, 2010.
- ^ Charles E. Mortimer: Chemistry - The basic knowledge of chemistry , Thieme 2003, ISBN 3-13-484308-0 .
- ↑ a b c Entry on benzoic acid. In: Römpp Online . Georg Thieme Verlag, accessed on December 11, 2011.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-38.
- ↑ Entry on Benzoic acid in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ Christian Wiegand: Origin and interpretation of important organic trivial names. In: Angewandte Chemie. A / 60. Born 1948 / No. 4th
- ↑ Klaus D. Christof, Renate Haass: Weihrauch: the scent of heaven. JH Röll Verlag, 2006, ISBN 978-3-89754-252-5 , pp. 49-50.
- ↑ Entry on benzoin. In: Römpp Online . Georg Thieme Verlag, accessed on February 9, 2012.
- ↑ ZZulV : Annex 5 (to Section 5, Paragraph 1 and Section 7) - additives that are approved for foodstuffs for preservation or as antioxidants .
- ↑ Federal Office for Consumer Protection and Food Safety : List of additives permitted for feed , Section 12: Acidity regulators . Retrieved June 27, 2015.
- ↑ Short article on Welt-Online from May 28, 2008.
- ↑ Opinion No. 013/2006 of the BfR of December 1, 2005: Indications of the possible formation of benzene from benzoic acid in food (PDF; 58 kB)
- ↑ Questions and Answers - BfR of December 16, 2013: Questions and answers about benzene in soft drinks and carrot juices .



