Magnesia pascoite
| Magnesia pascoite | |
|---|---|
| General and classification | |
| other names |
IMA 2007-025 |
| chemical formula |
|
|
Mineral class (and possibly department) |
Oxides (hydroxides, V [5,6] vanadates, arsenites, antimonites, bismuthites, sulfites, selenites, tellurites, iodates) |
|
System no. to Strunz and to Dana |
4.HC.05 ( 8th edition : IV / G.01-028 (Lapis system)) 47.02.01.02 |
| Similar minerals | Pascoite, hummerite, lasalite |
| Crystallographic Data | |
| Crystal system | monoclinic |
| Crystal class ; symbol | monoclinic prismatic; 2 / m |
| Space group | C 2 / m (No. 12) |
| Lattice parameters |
a = 19.8442 Å ; b = 9.9353 Å; c = 11.7372 Å β = 10.7149 ° |
| Formula units | Z = 2 |
| Frequent crystal faces | {302}, {401}, {112}, {113}, {11 1 }, {11 2 }, {22 3 }, {33 1 } |
| Twinning | no |
| Physical Properties | |
| Mohs hardness | ≈ 2.5 |
| Density (g / cm 3 ) | 2.43 (measured), 2.442 (calculated) |
| Cleavage | very perfect after {001} |
| Break ; Tenacity | shell-like with curved surfaces; brittle |
| colour | bright orange |
| Line color | yellow |
| transparency | transparent |
| shine | Diamond luster |
| Crystal optics | |
| Refractive indices |
n α = 1.769 n β = 1.802 n γ = 1.807 |
| Birefringence | δ = 0.038 |
| Optical character | biaxial negative |
| Axis angle | 2V = 45 ° (measured); 2V = 42 ° (calculated) |
| Pleochroism | clearly from Y = orange to X = Z = yellow |
| Other properties | |
| Chemical behavior | quickly soluble in cold, diluted HCl , slowly in H 2 O. Rapid decomposition, probably due to dehydration, when heated. |
Magnesiopascoite is a very rarely occurring mineral from the mineral class of the " oxides (as well as hydroxides , V [5,6] - vanadates , arsenites , antimonites, bismuthites, sulfites , selenites , tellurites and iodates )". It crystallizes in the monoclinic crystal system with the idealized chemical composition Ca 2 Mg (V 5+ 10 O 28 ) · 16H 2 O and is therefore chemically a water-containing magnesium - calcium - decavanadate , which belongs to the [6] sorovanadates (group vanadates).
The type locality of Magnesiopascoits is about 15 kilometers east of La Sal in the Lion Canyon located, abandoned today uranium - vanadium - deposit the "Blue Cap Mine" ( coordinates of the UV deposit Blue Cap Mine ) in the La Sal District (Paradox Valley District), San Juan Co. , Utah , USA .
The mineral is found at its type locality mainly in the form of parallel stacks of crystals, which vary in habit from tabular to isometric to prismatic. Their complex intergrown aggregates can reach sizes of several millimeters.
Etymology and history
As a result of the activities of the mineral collector Joe Marty from Salt Lake City in the area of the UV deposits around La Sal, a mineral was detected from the "Blue Cap Mine" and also from the nearby "Vanadium Queen Mine" which, in the first analyzes, did not match any other known phase was assigned.
After the determination of the required physical and optical properties and the chemical composition as well as the crystal structure by Anthony R. Kampf from the "Mineral Sciences Department" of the "Natural History Museum of Los Angeles County", Los Angeles / California , and Ian M. Steele from " Department of the Geophysical Sciences “of the University of Chicago , Chicago / Illinois , the mineral was presented to the International Mineralogical Association (IMA), which recognized it as a new mineral on 2007 under the provisional designation IMA 2007-025. In 2008, this mineral was first described scientifically by Anthony R. Kampf and Ian M. Steele in the Canadian science magazine " The Canadian Mineralogist " as Magnesiopascoit ( English Magnesiopascoite ). They named the mineral after its chemical composition with magnesium and its crystal-chemical relationship with Pascoit.
The type material (two cotypes) for magnesiopascoit is kept under catalog numbers 58610 and 58611 in the collection of the Natural History Museum of Los Angeles County in Los Angeles.
classification
Since the magnesiopascoit was only recognized as an independent mineral in 2008, it is not listed in the 8th edition of the mineral classification according to Strunz, which has been outdated since 1982 . In the "Lapis mineral directory", which was last updated in 2018 and which, out of consideration for private collectors and institutional collections, is still based on this classic system of Karl Hugo Strunz , the mineral was given the mineral and system no. IV / G.01-028 , which in the "Lapis system" corresponds to the section "Vanadium oxides (polyvanadates with V 4+ / 5+ )". There Magnesiopascoit together with Bluestreakit , Burroit , Gunterit , Huemulit , Hughesit , Hummerit , Kokinosit , Lasalit , Nashit , Pascoite , Postit , Rakovanit , Schindlerit , Sherwoodit , Wernerbaurit the group of "Gruppenvanadate".
The 9th edition of Strunz's mineral systematics, valid since 2001 and used by the International Mineralogical Association (IMA), assigns the magnesiopascoite to the mineral class of "oxides (as well as hydroxides, V [5,6] -vanadates, arsenites, antimonites, bismuthites, Sulfites, selenites, tellurites and iodates) "and there in the section of" V [5,6] Vanadates ". This is further subdivided according to the structure of the vanadate complexes, so that the mineral, according to its structure, can be found in the subsection "[6] -Group vanadates (Sorovanadates)", where together with Lasalit and Pascoit the Pascoit group with system no. 4.HC.05 forms.
The systematics of minerals according to Dana , which is mainly used in the English-speaking world , assigns the magnesiopascoit to the class of "phosphates, arsenates and vanadates" and there to the category of "vanadium oxy salts". There he can be found together with Pascoit in the unnamed group 47.02.01 within the subdivision " Vanadium Oxy Salts (VmOn) ".
Chemism
Four microprobe analyzes on magnesiopascoite from the type locality yielded mean values of 7.78% CaO; 2.67% MgO; 0.23% ZnO; 0.05% CoO; 71.32% V 2 O 5 and 21.94% H 2 O (calculated from the crystal structure) (sum = 103.99%). On the basis of ten vanadadium atoms per formula unit (apfu), with 44 oxygen atoms apfu and sufficient hydrogen (for charge balance ), the empirical formula Ca 1.77 (Mg 0.85 Zn 0.04 Co 0.01 ) ( H 2 O) 15.34 (H 3 O) 0.66 (V 10 O 28 ) calculated. This was idealized to Ca 2 Mg (V 5+ 10 O 28 ) · 16H 2 O, which contents of 2.98% MgO; 8.31% CaO; 67.35% V 2 O 5 and 21.35% H 2 O (total = 100.00%) required.
Magnesiopascoite is the only mineral that has the combination of elements Mg – Ca – V – O – H. The vanadate mineral gottlobite , CaMg (VO 4 , AsO 4 ) (OH), whose vanadate complexes can be substituted by arsenate complexes, has a very similar chemical composition . Magnesiopascoite is the Mg-dominant (and slightly less watery) analogue of the Ca-dominated pascoite and the Ca 2 Mg analogue of the K 2 Mg 2 -dominant hummerite, K 2 Mg 2 (V 10 O 28 ) · 16H 2 O. All of these Minerals also contain the [V 10 O 28 ] 6– -decavanadate polyanion, but have different cation occupations with alkali or alkaline earth ions or both and different water of crystallization contents.
Chemically similar, however, are u. a. Alpeit , Ca 4 Mn 3+ 2 Al 2 (Mn 3+ Mg) (SiO 4 ) 2 (Si 3 O 10) (V 5+ O 4 ) (OH) 6 ; Kannanite , Ca 4 Al 4 (AlMg) (VO 4 ) (SiO 4 ) 2 (Si 3 O 10 ) (OH) 6 ; Lumsdenite , NaCa 3 Mg 2 (As 3+ V 4+ 2 V 5+ 10 As 5+ 6 O 51 ) • 45H 2 O; Poppiite , Ca 2 (V 3+ , Fe 3+ , Mg) (V 3+ , Al) 2 (Si 2 O 7 ) (SiO 4 ) (OH, O) 2 · H 2 O; vanadium-containing adelite , CaMg ([As, V] O4) (OH); Vanadium pargasite , NaCa 2 (Mg 4 V) (Al 2 Si 6 ) O 22 (OH) 2 ; as well as the as yet undescribed mineral phase "UM1979-21-SiO: AlHNaV", (Na, Ca) 0.73 (V, Mg, Fe) 2 (Si, Al, V) 4 O 10 (OH) 2 · nH 2 O.
Crystal structure
Magnesiopascoite crystallizes in the monoclinic crystal system in the space group C 2 / m (space group no.12 ) with the lattice parameters a = 19.8442 Å , b = 9.9353 Å, c = 11.7372 Å and β = 10.7149 ° as well two formula units per unit cell .
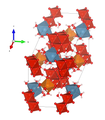
The crystal structure of the Magnesiopascoit (compare the adjacent structural representations) is very similar to that of other representatives of the Pascoit group. It consists of the structural unit (or the anionic complex) of the decavanadate polyanion (or oxyanion) [V 10 O 28 ] 6– and an interstitial, hydrogenated, cationic complex. The decavanadate moiety consists of ten highly distorted V 5+ O 6 - octahedra with common edges. Eight of the ten octahedra [V2 (× 2), V3 (× 2) and V4 (× 4)] in the magnesiopascoit and also in the pascoit are trans to the vanadyl bond through a short vanadyl bond, four medium-length equatorial bonds and a long bond marked. The other two octahedra [V1 (× 2)] in the magnesiopascoit and pascoit have two short trans bonds and four medium-length equatorial bonds. The marked variations in bond length are consistent with the strong electrostatic repulsion between the V 5+ cations.
| Crystal structure of magnesiopascoite |
|
|
| Color legend: __ V __ Mg __ Ca __ O __ H |
In magnesiopascoit, the interstitial complex has the composition {Ca 2 Mg (H 2 O) 16 } 6+ and is made up of Mg (H 2 O) 6 octahedra and seven-coordinate CaO 2 (H 2 O) 5 groups (including of the two oxygen atoms from the decavanadate complex to which Ca is bound). In the Pascoit, however, the interstitial complex consists of {Ca 2 Ca (H 2 O) 17 } 6+ and is formed by seven-coordinate Ca (H 2 O) 7 and CaO 2 (H 2 O) 5 groups. The only significant difference between the structures of magnesiopascoite and pascoite relates to the interstitial complex in the structures and there to the coordination of the two non-equivalent cation positions forming the respective complex. While in the pascoit structure the Ca1 position is coordinated with seven H 2 O molecules - four in fully occupied positions and three in half occupied positions - in the structure of the magnesiopascoite the Mg with six H 2 O molecules - and all of these in fully occupied positions - coordinated, forming an almost perfect octahedron. In both cases the cations are only bound to H 2 O molecules, which in turn are only linked to oxygen atoms of the interstitial complex via hydrogen bonds . The coordination around the other cation position - Ca2 in pascoit and Ca in magnesiopascoit - are both sevenfold and differ only in that a pair of bound oxygen positions (O12) is shared in the pascoit and not in the magnesiopascoit. In both cases Ca is connected to the two oxygen atoms O1 and O5 of the decavanadate complex and five H 2 O molecules (O12 (× 2), O14 (× 2) and O15).
properties
morphology
At its type locality, magnesiopascoite forms parallel stacks of crystals, which vary in habit from tabular to isometric to prismatic. They can be fused into complex aggregates that can reach several millimeters in size. The basic pinacoid {001} is decisive for the costume , in addition there are countless small surface shapes in the zones [110] and [1 1 0], which lead to a clear striation of the crystals arranged at 120 ° to each other and at the same time simulate a hexagonal symmetry . The shapes in the mentioned zones include {302}, {401}, {112}, {113}, {11 1 }, {11 2 }, {22 3 } and {33 1 }. Twins are not known.
physical and chemical properties
Magnesiopascoit crystals are colored bright orange, their stroke color is indicated with yellow. The surfaces of the transparent crystals show a characteristic diamond-like sheen . Magnesiopascoit has a high light refraction (n α = 1.769; n β = 1.802; n γ = 1.807) and a high birefringence (δ = 0.038) corresponding to this diamond luster . The optically biaxially negative Magnesiopascoit has an optical axis angle 2V of 45 ° (measured) or 42 ° (calculated) and a strong dispersion with r <v. In transmitted light, the mineral shows orange to yellowish tones with a clear pleochroism from Y = orange to X = Z = yellow.
Magnesiopascoit dissolves quickly in cold, dilute hydrochloric acid , HCl, but much more slowly in H 2 O. When heated, it decomposes quickly, probably through dehydration . There are no statements on possible fluorescence in long or short wave UV light .
Overall, magnesiopascoite looks very similar to the other minerals of the Pascoite group such as pascoite, hummerite and lasalite, which makes a purely visual identification problematic. For this reason, magnesia pascoite has often been overlooked and is therefore much more widespread.
Education and Locations
As a very rare mineral formation, the magnesiopascoite has so far (as of 2019) only been described by six sites. The type locality for Magnesiopascoit is about 15 kilometers east of La Sal in the Lion Canyon located, abandoned today uranium - vanadium - deposit the "Blue Cap Mine" in La Sal District (Paradox Valley District), San Juan Co. , Utah , USA . The maximum length of the mines is 600 feet with numerous ore falls , punctures and stretches to the ore bodies on either side of the adit . Many of the ore bodies are located in "paleocannels".
The “Blue Cap Mine” is a deposit with finely divided (disseminated) ore that has displaced organic components such as wood and / or bones in the “Salt Wash Member” of the Morrison Formation . Kohliges material forms a layer in this reducing environment, and is responsible for the precipitation of uranium and vanadium oxides (such. As uraninite , Corvusit and montroseite ) from solutions. Magnesiopascoite is a characteristic secondary mineral and, similar to rossite and martyite , was only formed at its type locality after mining activities through leaching and oxidation of vanadium oxides by circulating groundwater. Typical accompanying minerals of the magnesiopascoite are gypsum , rossite, pyrite , montroseite and martyite.
In addition to the type locality, there are a few other sites for magnesiopascoit. This includes:
- the "Packrat Mine" at Gateway in the Gateway District, both in Mesa County , Colorado , USA
- the "Blue Streak Mine" in the Bull Canyon District, Montrose County , Colorado, USA
- the “Opera Box Mine” (Aztec Mine) in Gypsum Valley, Montrose Co., Colorado, USA
- the "Vanadium Queen Mine", both at La Sal in the La Sal District (Paradox Valley District), San Juan Co. , Utah , USA
- the "Firefly-Pigmay Mine" in the La Sal Quadrangle, San Juan Co., Utah, USA
Locations for magnesia pascoit from Germany , Austria and Switzerland are therefore unknown.
use
Due to its rarity, magnesia pascoite is only of interest to the collector of minerals.
See also
literature
- Anthony R. Kampf, Ian M. Steele: Magnesiopascoite, a new member of the pascoite group: description and crystal structure . In: The Canadian Mineralogist . tape 46 , no. 3 , 2008, p. 679–686 , doi : 10.3749 / canmin.46.3.679 (English, rruff.info [PDF; 917 kB ; accessed on March 3, 2019]).
- Magnesiopascoite . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 ( [2] [PDF; 117 kB ; accessed on March 3, 2019]).
Web links
- Mineral Atlas: Magnesiopascoite (Wiki)
- Magnesiopascoite. In: mindat.org. Hudson Institute of Mineralogy, accessed March 3, 2019 .
- David Barthelmy: Magnesiopascoite Mineral Data. In: webmineral.com. Accessed March 3, 2019 .
- Magnesiopascoite search results. In: rruff.info. Database of Raman spectroscopy, X-ray diffraction and chemistry of minerals (RRUFF), accessed March 3, 2019 .
- American-Mineralogist-Crystal-Structure-Database - Magnesiopascoite. In: rruff.geo.arizona.edu. Accessed March 3, 2019 .
Individual evidence
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao Anthony R. Kampf, Ian M. Steele: Magnesiopascoite, a new member of the pascoite group: description and crystal structure . In: The Canadian Mineralogist . tape 46 , no. 3 , 2008, p. 679–686 , doi : 10.3749 / canmin.46.3.679 (English, rruff.info [PDF; 917 kB ; accessed on March 3, 2019]).
- ^ IMA / CNMNC List of Mineral Names; November 2018 (PDF 1.65 MB)
- ↑ Stefan Weiß: The large Lapis mineral directory. All minerals from A - Z and their properties. Status 03/2018 . 7th, completely revised and supplemented edition. Weise, Munich 2018, ISBN 978-3-921656-83-9 .
- ↑ a b c d Magnesiopascoite. In: mindat.org. Hudson Institute of Mineralogy, accessed March 3, 2019 .
- ↑ Magnesiopascoite . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 ( [1] [PDF; 117 kB ; accessed on March 3, 2019]).
- ↑ Localities for Magnesiopascoite. In: mindat.org. Hudson Institute of Mineralogy, accessed March 3, 2019 .
- ↑ List of locations for magnesiopascoite at the Mineralienatlas and at Mindat (accessed on March 3, 2019)
- ↑ Description of Blue Cap Mine. In: mindat.org. Hudson Institute of Mineralogy, accessed March 3, 2019 .
- ↑ Anthony Kampf, Barbara P. Nash, Joe Marty, John M. Hughes: Mesaite, CaMn 2+ 5 (V 2 O 7 ) 3 12H 2 O, a new vanadate mineral from the Packrat mine, near Gateway, Mesa County, Colorado, USA . In: Mineralogical Magazine . tape 81 , no. 2 , 2017, p. 319–327 , doi : 10.1180 / minmag.2016.080.095 (English).
- ^ Anthony R. Kampf, Joe Marty, Barbara P. Nash, Jakub Plášil, Anatoly V. Kasatkin, Radek Škoda: Calciodelrioite, Ca (VO 3 ) 2 (H 2 O) 4 , the Ca analogue of delrioite, Sr (VO 3 ) 2 (H 2 O) 4 . In: Mineralogical Magazine . tape 76 , no. 7 , 2012, p. 2803–2817 , doi : 10.1180 / minmag.2012.076.7.12 (English, researchgate.net [PDF; 2.5 MB ; accessed on February 25, 2019]).
- ^ Anthony R. Kampf, John M. Hughes, Joe Marty, Barbara P. Nash, Yu-Sheng Chen, Ian M. Steele: Bluestreakite, K 4 Mg 2 (V 4+ 2 V 5+ 8 O 28 ) · 14H 2 O, a new mixed-valence decavanadate mineral from the Bluestreak Mine, Montrose County, Colorado: crystal structure and descriptive mineralogy . In: The Canadian Mineralogist . tape 52 , no. 6 , 2014, p. 1007-1018 , doi : 10.3749 / canmin.1400072 (English, researchgate.net [PDF; 389 kB ; accessed on February 25, 2019]).