Huemulit
| Huemulit | |
|---|---|
| Deep orange huemulite crystals on a dark matrix from the "West Sunday Mine", Slick Rock District, San Miguel County, Colorado, USA | |
| General and classification | |
| other names |
IMA 1965-012 |
| chemical formula |
|
|
Mineral class (and possibly department) |
Oxides (hydroxides, V [5,6] vanadates, arsenites, antimonites, bismuthites, sulfites, selenites, tellurites, iodates) |
|
System no. to Strunz and to Dana |
4.HG.10 ( 8th edition : IV / F.08) 02.47.03.01 |
| Crystallographic Data | |
| Crystal system | triclinic |
| Crystal class ; symbol | triclinic pinacoidal; 1 |
| Space group | P 1 (No. 2) |
| Lattice parameters |
a = 9.0453 Å ; b = 11.3337 Å; c = 11.7372 Å, α = 105.223 °; β = 97.383 °; γ = 100.790 ° |
| Formula units | Z = 1 |
| Frequent crystal faces | {100}, {110}, {010}, {001} |
| Physical Properties | |
| Mohs hardness | "Soft" (natural material), 2.5 to 3 (recrystallized) |
| Density (g / cm 3 ) | 2.36 (measured) |
| Cleavage | very perfectly parallel (001), less well parallel (010) (recrystallized) |
| Break ; Tenacity | not given; brittle (recrystallized) |
| colour | yellow-orange to orange (natural); yellowish orange to reddish orange (recrystallized) |
| Line color | yellow (natural); yellowish orange (recrystallized) |
| transparency | transparent |
| shine | matte (natural); Glass to semi-diamond luster (recrystallized) |
| Crystal optics | |
| Refractive indices |
n α = 1.679 n β = 1.734 n γ = 1.742 |
| Birefringence | δ = 0.063 |
| Optical character | biaxial negative |
| Axis angle | 2V = 20 ° to 25 ° |
| Pleochroism | clearly from X = light yellow to Y = golden yellow to Z = yellowish orange |
| Other properties | |
| Chemical behavior | Easily soluble in cold water, melts at 500 ° C to a red liquid |
Huemulite is a very rare mineral from the mineral class of " oxides (as well as hydroxides , V [5,6] - vanadates , arsenites , antimonites, bismuthites, sulphites , selenites , tellurites and iodates )". It crystallizes in the triclinic crystal system with the idealized chemical composition Na 4 Mg (V 10 O 28 ) · 24H 2 O and is therefore chemically a water-containing sodium - magnesium - decavanadate , which belongs to the "unclassified vanadium [V] oxides".
The type locality of Huemulits is 40 km southwest of the city of Malargüe in Pampa Amarilla District located, abandoned today uranium - deposit of "Mina Huemul" ( coordinates of the underground deposit Mina Huemul ) malargüe department , province of Mendoza , Argentina .
The mineral can be found at its type locality mainly in the form of aggregates of fine fibers and thin films as well as kidney and massive. Huemulite from the "West Sunday Mine" in San Miguel Co. , Colorado , USA , on the other hand , forms groups of idiomorphic crystals with a maximum size of 0.4 mm.
Etymology and history
As early as 1959, Victorio Angelelli of the Argentine Comissión Nacional de Energía Atómica (CNEA) was able to recover a mineral in the area of level −18 of the ore body "Agua Botada" of the "Mina Huemul" near Malargüe, Department Malargüe, Province of Mendoza, Argentina, which Enrique Linares identified as a possible U and / or V secondary mineral. The first optical and X-ray diffractometric analyzes, however, showed data and a diffractogram that did not match any of the other known UV minerals - a new phase was therefore present. Other identical material was also found in the neighboring ore bodies “Huemul” and “Agua Botada Sur”.
After the required physical and optical properties and the chemical composition as well as the crystal structure had been determined by a team of scientists led by Carlos E. Gordillo from the CNEA, the mineral was presented to the International Mineralogical Association (IMA), which gave it the provisional name on May 19, 1965 IMA 1965-012 recognized as a new mineral. In 1966, the first scientific description of this mineral was carried out by a team of Argentine and US scientists with Carlos E. Gordillo, Enrique Linares, Roberto O. Toubes and Horace Winchell in the US scientific journal " The American Mineralogist " as Huemulit ( English Huemulite ). They named the mineral after the Mina Huemul , the most important uranium deposit in the Malargüe area.
The type of material for Huemulit is in the collection of the catalog number NMNH-120076 Smithsonian Institution belonging to National Museum of Natural History , Washington, DC , kept.
classification
In the now outdated, but partly still in use 8th edition of the mineral classification according to Strunz , the huemulite belonged to the mineral class of "oxides and hydroxides" and there to the department of "vanadium hydroxides", where it together with lobsterite and pascoite (as well as magnesiopascoite and rakovanite ) the Pascoit-Hummerit group with system no. IV / F.08 .
The 9th edition of Strunz's mineral systematics, which has been in effect since 2001 and is used by the International Mineralogical Association (IMA), assigns the huemulite to the mineral class of "oxides (as well as hydroxides, V [5,6] -vanadates, arsenites, antimonites, bismuthites, Sulfites, selenites, tellurites and iodates) "and there in the section of" V [5,6] Vanadates ". This is, however, further subdivided according to the structure of the vanadate complexes, so that the mineral can be found in the sub-section “Unclassified Vanadium Oxides” according to its structure, where it is the only member of the unnamed group 4.HG.10 .
The systematics of minerals according to Dana , which is mainly used in the English-speaking world , assigns the huemulite to the class of "phosphates, arsenates and vanadates" and there to the category of "vanadium oxy salts". There he is to be found as the only member of the unnamed group 47.02.03 within the subdivision " Vanadium-Oxysalze (VmOn) ".
Chemism
The first chemical analysis on Huemulit yielded 40.21% V 2 O 5 ; 0.02% MnO; 1.18% MgO; 3.53% CaO; 3.94% Na 2 O; 0.52% K 2 O; 8.80% H 2 O + ; 12.00% H 2 O - ; 4.45% SO 3 and 25.43% residue insoluble in water (total 100.08%). The contents of CaO and SO 3 correspond to mechanically added gypsum . Analyzes of huemulite dissolved and recrystallized in water showed 59.8% V 2 O 5 ; 3.0% MgO; 8.4% Na 2 O and 29.2% H 2 O + (total 100.04%). The idealized composition Na 4 Mg (V 10 O 28 ) · 24H 2 O was determined from the analysis of the recrystallized Huemulite , the contents of 60.39% V 2 O 5 ; 2.68% MgO; 8.24% Na 2 O and 28.69% H 2 O + (total 100.00%) are required.
Of all known minerals, only lasalite , Na 2 Mg 2 (V 10 O 28 ) · 20H 2 O, has the same combination of elements Na – Mg – V – O – H as huemulite - it is the analogue of the Lasalite. Ammoniolasalite , (NH 4 ) 2 Mg 2 (H 2 O) 20 [V 10 O 28 ], pascoite , Ca 3 (V 10 O 28 ) · 17H 2 O, and lobsterite , K 2 Mg 2 (V 10 O 28 ) · 16H 2 O, also contain the [V 10 O 28 ] 6– -decavanadate polyanion, but have a different cation occupation and different water of crystallization.
Chemically similar, however, are u. a. Bicapite , [KNa 2 Mg 2 (H 2 O) 25 ] [H 2 PV 5+ 12 O 40 (V 5+ O) 2 ]; Chernykhit , (Ba, Na) (V 3+ , Al, Mg) 2 ((Si, Al) 4 O 10 ) (OH) 2 ; Lumsdenite , NaCa 3 Mg 2 (As 3+ V 4+ 2 V 5+ 10 As 5+ 6 O 51 ) • 45H 2 O; Oxy-vanadium dravite , Na (V) 3 (V 4 Mg 2 ) Si 6 O 18 (BO 3 ) 3 (OH) 3 O; Vanadio-Oxy-Chromium-Dravite , Na (V) 3 (Cr 4 Mg 2 ) (Si 6 O 18 ) (BO 3 ) 3 (OH) 3 O; Vanadio-oxy-dravite , NaV 3 (Al 4 Mg 2 ) (Si 6 O 18 ) (BO 3 ) 3 (OH) 3 O; and vanadium pargasite , NaCa 2 (Mg 4 V) (Al 2 Si 6 ) O 22 (OH) 2 ; as well as the as yet undescribed mineral phase "UM1979-21-SiO: AlHNaV", (Na, Ca) 0.73 (V, Mg, Fe) 2 (Si, Al, V) 4 O 10 (OH) 2 · nH 2 O.
Crystal structure
Huemulite crystallizes in the triclinic crystal system in the space group P 1 (space group no. 2) with the lattice parameters a = 9.0453 Å , b = 11.3337 Å, c = 11.7372 Å, α = 105.223 °, β = 97.383 ° and γ = 100.790 ° and one formula unit per unit cell .
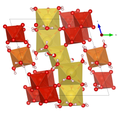
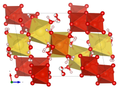
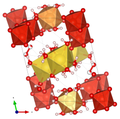
| Crystal structure of huemulite |
|
|
| Color legend: __ V __ Mg __ Na __ O __ H |
The crystal structure of the huemulite (compare the adjacent structural representations) consists of decavanadate polyanions (or oxyanions) [V 10 O 28 ] 6– , which are connected to one another via an interstitial complex, which is made up of isolated [Mg (H 2 O) 6 ] 2+ - octahedra and a cationic [Na 4 (H 2 O) 14 ] 4+ consist group. Both units define a zigzag endless chain. There are also four isolated H 2 O groups, two of which are positionally disordered. All but four hydrogen atoms could be located; they show a network of hydrogen bonds , which also connects the interstitial complex with the structural unit and in this way stabilizes the atomic arrangement.
Huemulit is chemically and structurally similar to the members of a family of synthetic materials with the formula Na 4 MV 10 O 28 · 23H 2 O (with M = Ni 2+ , Mg).
properties
morphology
At its type locality, huemulite is found in grape-like masses, in microscopic fibers, in rounded, thin film-forming masses or in the pores of the sandstone, which is the adjacent rock. Idiomorphic huemulite crystals from the "West Sunday Mine" in San Miguel County in Colorado / USA reach a maximum size of 0.4 mm and come together in groups. Huemulite dissolved in water and recrystallized again forms tabular crystals with the other planar forms {100}, {010} and {110} after the basic pinacoid {001} (compare the crystal drawing opposite). Additional, non-indexed surface shapes can be seen in SEM images.
physical and chemical properties
Most of the physico-optical and chemical properties of the huemulite of the first description were determined on the huemulite dissolved in water and then recrystallized by evaporation.
Huemulite crystals are bright orange in color. Natural aggregates are yellow-orange to orange, while recrystallized material is yellowish-orange to reddish-orange in color, depending on the size of the crystals. The line color of natural aggregates is indicated with yellow, that of recrystallized material with yellowish orange. The surfaces of the transparent crystals show a characteristic glass-like to semi-diamond-like sheen , while natural, fine-grained aggregates are matt. Huemulit has a high light refraction (n α = 1.679; n β = 1.734; n γ = 1.742) and a high birefringence (δ = 0.063) corresponding to this glass to semi-diamond gloss . The optically biaxially negative huemulite has an optical axis angle 2V of 20 ° to 25 ° and a strong dispersion with r> v. In transmitted light, the mineral shows yellowish to yellowish orange tones with a clear pleochroism from X = light yellow to Y = golden yellow to Z = yellowish orange.
Recrystallized Huemulite has a very perfect cleavage parallel (001) and a less good cleavage parallel (010). A break is not indicated, recrystallized Huemulite is brittle. Recrystallized huemulite has a Mohs hardness of 2.5 to 3 and is one of the soft to medium-hard minerals that can be scratched with a copper coin just as easily as the reference mineral calcite (hardness 3). The measured density for natural huemulite aggregates is 2.39 g / cm³, for huemulite crystals from the "West Sunday Mine" 2.232 g / cm³.
Huemulite is easily soluble in cold water , H 2 O. At 500 ° C the mineral melts into a red liquid. Huemulit has neither in the short nor in the long-wave UV light , a fluorescent on.
Education and Locations
As a very rare mineral formation, the Huemulite has so far (as of 2019) only been described by about twenty sites. The type locality for Huemulit is 40 km southwest of the city of Malargüe in Pampa Amarilla District located, abandoned today uranium - deposit of "Mina Huemul" malargüe department , province of Mendoza , Argentina . The mineral is also found in the neighboring ore bodies "Agua Botada" and "Agua Botada Sur" of this deposit.
In the "Mina Huemul" is a sedimentary uranium - deposit , the mineralization as impregnation in sandstones and conglomerates sitting. In the area of the weathering zone, these contain asphalt and carbonate material with uranophane , copper carbonates and iron oxides, in their unweathered, primary area, however, carnotite and pitchblende , associated with pyrite and copper sulfides ( chalcopyrite , bornite and chalcosine ). The ore bodies of the deposit, which vary in size and shape, reach a total volume of several thousand to several tens of thousands of tons of ore. The U 3 O 8 contents vary between 0.20 and 0.30% and occasionally more.
The ore body "Huemul" has dimensions between 60 m and 100 m in strike and 310 m in fall and an average thickness of 1.15 m. The mineralization of the pending ore consists mainly of carnotite and tyuyamunite, as well as phosphuranylite , uranophane and autunite , which are accompanied by malachite , azurite and iron hydroxides. In the lower areas of the mineralized body, asphalt- like pyrobitumen is the carrier of uranium mineralization ; it also contains pitchblende and minor amounts of small grains of metal sulfides such as pyrite, chalcopyrite, bornite, pyrrhotite , chalcosine, galena and sphalerite . In the mineralized area as well as above lenticular masses made of pure clay, sandstone lenses occur that are heavily impregnated with asphalt-like pyrobitumen with U 3 O 8 contents of 2 to 3%.
Huemulite is a typical secondary mineral and was only formed at its type locality after the Cu – U deposits in the sandstones and conglomerates had been excavated. The vanadium is likely to come from the associated asphalt-like pyrobitumen. Typical accompanying minerals of the huemulite, which can be easily recognized by its bright color, are hummerite and rossite as well as thenardite , gypsum and epsomite .
In addition to the type locality, there are a few other sites for Huemulite. This includes:
- the "Pit No. 21 “(shaft No. 21) in Háje near Hřiměždice not far from Příbram , Středočeský kraj , Czech Republic
- the "Liebenbergite slag dump " not far from the village of Agios Konstantinos (Kamariza) ( modern Greek Αγ. Κωνσταντίνος (Καμάριζα) ) not far from Plaka, mining district Lavrion , Attica region , Greece
- the "Mina Eureka", Castell-estaó, La Torre de Cabdella, La Vall Fosca, El Pallars Jussà, Lleida Province , Catalonia , Spain
- the Cu, Co and Ni vanadium-uranium concretions of the Red Bed outcrop " Littleham Bay " near Budleigh Salterton , East Devon District , Devon , England , United Kingdom
- the "Blue Streak Mine" in the Bull Canyon District, Montrose County , Colorado , USA
- the "St Jude Mine" in the Gypsum Valley and the "West Sunday Mine," both in the Slick Rock District, San Miguel Co. , Colorado, USA
- "Suncup No. 2 “(Puckett) in the Uravan District, San Miguel Co., Colorado, USA
- the "Bisoni Prospect" (McCulloughs Butte Prospect) in the Fish Creek District, Eureka County , Nevada , USA
- the "VanNavSan Claim" (Van Nav Sand claim), Fish Creek Range, Gibellini District, Eureka Co., Nevada, USA
- the "Gibellini Mine" (Niganz mine; Black Iron mine) and "The Fish", both in the Gibellini District, Eureka Co., Nevada, USA
- the "Little Eva Mine" at Yellow Cat Mesa, Thompsons District (SE Thomsons), Grand County , Utah , USA
- the "D-Day No. 4 Mine “, D-Day Mine Group, Thompsons District (SE Thomsons), Grand Co. , Utah, USA
Sites for huemulite from Germany , Austria and Switzerland are therefore unknown.
use
Due to its rarity, huemulite is only of interest to mineral collectors.
See also
literature
- Carlos E. Gordillo, Enrique Linares, Roberto O. Toubes, Horace Winchell: Huemulite, Na 4 MgV 10 O 28 · 24H 2 O, a new hydrous sodium a magnesium vanadate from Huemul mine, Mendoza province, Argentina . In: The American Mineralogist . tape 51 , no. 1-2 , 1966, pp. 1–13 (English, rruff.info [PDF; 767 kB ; accessed on February 25, 2019]).
- Enrique Linares, Carlos E. Gordillo, Roberto O. Toubes, Horace Winchell: Huemulita, Na 4 MgV 10 O 28 · 24H 2 O, un nuevo vanadato hidratado de sodio y magnesio, de la mina Huemul Mendoza, Argentina . In: Comisión nacional de energía atómica // República Argentina . tape 189 , 1967, p. 1–21 (Spanish, gob.ar [PDF; 597 kB ; accessed on February 25, 2019]).
- Fernando Colombo, Ricardo Baggio, Anthony R. Kampf: The crystal structure of the elusive huemulite . In: The Canadian Mineralogist . tape 49 , no. 3 , 2011, p. 849–864 , doi : 10.3749 / canmin.49.3.849 (English, rruff.info [PDF; 1.9 MB ; accessed on February 25, 2019]).
- Huemulite . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 ( handbookofmineralogy.org [PDF; 71 kB ; accessed on February 25, 2019]).
Web links
- Mineral Atlas: Huemulite (Wiki)
- Huemulite. In: mindat.org. Hudson Institute of Mineralogy, accessed February 25, 2019 .
- David Barthelmy: Huemulite Mineral Data. In: webmineral.com. Retrieved February 25, 2019 .
- Huemulite search results. In: rruff.info. Database of Raman spectroscopy, X-ray diffraction and chemistry of minerals (RRUFF), accessed on February 25, 2019 .
- American-Mineralogist-Crystal-Structure-Database - Huemulite. In: rruff.geo.arizona.edu. Retrieved February 25, 2019 .
Individual evidence
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai Carlos E. Gordillo, Enrique Linares, Roberto O. Toubes, Horace Winchell: Huemulite, Na 4 MgV 10 O 28 · 24H 2 O, a new hydrous sodium a magnesium vanadate from Huemul mine, Mendoza province, Argentina . In: The American Mineralogist . tape 51 , no. 1-2 , 1966, pp. 1–13 (English, rruff.info [PDF; 767 kB ; accessed on February 25, 2019]).
- ^ IMA / CNMNC List of Mineral Names; November 2018 (PDF 1.65 MB)
- ^ Hugo Strunz , Ernest H. Nickel : Strunz Mineralogical Tables. Chemical-structural Mineral Classification System . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 257 .
- ↑ a b c d e f g h i j k Fernando Colombo, Ricardo Baggio, Anthony R. Kampf: The crystal structure of the elusive huemulite . In: The Canadian Mineralogist . tape 49 , no. 3 , 2011, p. 849–864 , doi : 10.3749 / canmin.49.3.849 (English, rruff.info [PDF; 1.9 MB ; accessed on February 25, 2019]).
- ↑ Huemulite . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 ( handbookofmineralogy.org [PDF; 71 kB ; accessed on February 25, 2019]).
- ↑ a b c d e f Huemulite. In: mindat.org. Hudson Institute of Mineralogy, accessed February 25, 2019 .
- ↑ Localities for Huemulite. In: mindat.org. Hudson Institute of Mineralogy, accessed February 25, 2019 .
- ↑ List of localities for Lasalite in the Mineralienatlas and Mindat (accessed on February 24, 2019)
- ^ A b Description of Mina Huemul. In: mindat.org. Hudson Institute of Mineralogy, accessed February 25, 2019 .
- ↑ Jiří Sejkora, Jiří Litochleb: secondary minerals from the ore zone Příbram . In: Lapis . tape 28 , no. 7/8 , 2003, p. 65 .
- ^ Anthony R. Kampf, John M. Hughes, Joe Marty, Barbara P. Nash, Yu-Sheng Chen, Ian M. Steele: Bluestreakite, K 4 Mg 2 (V 4+ 2 V 5+ 8 O 28 ) · 14H 2 O, a new mixed-valence decavanadate mineral from the Bluestreak Mine, Montrose County, Colorado: crystal structure and descriptive mineralogy . In: The Canadian Mineralogist . tape 52 , no. 6 , 2014, p. 1007-1018 , doi : 10.3749 / canmin.1400072 (English, researchgate.net [PDF; 389 kB ; accessed on February 25, 2019]).
- ^ Anthony R. Kampf, Joe Marty, Barbara P. Nash, Jakub Plášil, Anatoly V. Kasatkin, Radek Škoda: Calciodelrioite, Ca (VO 3 ) 2 (H 2 O) 4 , the Ca analogue of delrioite, Sr (VO 3 ) 2 (H 2 O) 4 . In: Mineralogical Magazine . tape 76 , no. 7 , 2012, p. 2803–2817 , doi : 10.1180 / minmag.2012.076.7.12 (English, researchgate.net [PDF; 2.5 MB ; accessed on February 25, 2019]).
- ↑ Ray L. Frost, Kristy L. Erickson, Matt L. Weier, Onuma Carmody: Raman and infrared spectroscopy of selected vanadates . In: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy . 61A, no. 5 , 2005, p. 829-834 , doi : 10.1016 / j.saa.2004.06.006 (English).
- ↑ John M. Hughes, William S. Wise, Mickey E. Gunter, John P. Morton, John Rakovan: Lasalite, Na 2 Mg 2 [V 10 O 28 ] · 20H 2 O, a new decavanadate mineral species from the Vanadium Queen mine, La Sal District, Utah: Description, atomic arrangement, and relationship to the pascoite group of minerals . In: The Canadian Mineralogist . tape 46 , no. 5 , 2008, p. 1365–1372 , doi : 10.3749 / canmin.46.5.1365 (English, rruff.info [PDF; 1.8 MB ; accessed on February 24, 2019]).
- ↑ Anatoly V. Kasatkin, Jakub Plášil, Joseph Marty, Atali Al Agakhanov, Dimitrii Ilyich Belakovskiy, Inna S. Lykova: Nestolaite, CaSeO 3 · H 2 O, a new mineral from the Little Eva mine, Grand County, Utah, USA . In: Mineralogical Magazine . tape 78 , no. 3 , 2014, p. 497–505 , doi : 10.1180 / minmag.2014.078.3.02 (English).