Lasalit
| Lasalit | |
|---|---|
| Light orange lasalite crystals from the "Vanadium Queen Mine", La Sal Creek Canyon, Paradox Valley District, Paradox Valley, San Juan Co., Utah, USA (field of view: 6 mm) | |
| General and classification | |
| other names |
IMA 2007-005 |
| chemical formula |
|
|
Mineral class (and possibly department) |
Oxides (hydroxides, V [5,6] vanadates, arsenites, antimonites, bismuthites, sulfites, selenites, tellurites, iodates) |
|
System no. to Strunz and to Dana |
4.HC.05 47.02.07.01 |
| Similar minerals | Pascoite , Magnesiopascoit , Huemulit , Hummerit , Rakovanit |
| Crystallographic Data | |
| Crystal system | monoclinic |
| Crystal class ; symbol | monoclinic prismatic; 2 / m |
| Space group | C 2 / c (No. 15) |
| Lattice parameters |
a = 23.9019 Å ; b = 10.9993 Å; c = 17.0504 Å β = 118.284 ° |
| Formula units | Z = 4 |
| Frequent crystal faces | {100}, {010}, {001}, { 1 11}, {111} |
| Twinning | no |
| Physical Properties | |
| Mohs hardness | 1 |
| Density (g / cm 3 ) | 2.38 (measured); 2.362 (calculated) |
| Cleavage | no |
| Break ; Tenacity | not given; very brittle |
| colour | yellow to yellow-orange |
| Line color | yellow |
| transparency | transparent |
| shine | Diamond luster |
| Crystal optics | |
| Refractive indices |
n α = 1.743 n β = 1.773 n γ = 1.780 |
| Birefringence | δ = 0.037 |
| Optical character | biaxial negative |
| Axis angle | 2V = 32 ° (at 550 nm), 2V = 43 ° (589 nm), 2V = 43 ° (650 nm) (measured at three different wavelengths) |
| Pleochroism | clearly from X = light greenish yellow to Y = light yellow to Z = light brown |
| Other properties | |
| Chemical behavior | easily soluble in water |
| Special features | Crystals dehydrate to a yellow powder; also very rapid dehydration under the electron beam |
Lasalite is a very rarely occurring mineral from the mineral class of " oxides (as well as hydroxides , V [5,6] - vanadates , arsenites , antimonites, bismuthites, sulfites , selenites , tellurites and iodates )". It crystallizes in the monoclinic crystal system with the idealized chemical composition Na 2 Mg 2 (V 10 O 28 ) · 20H 2 O and is therefore chemically seen a hydrous sodium - magnesium - decavanadate , the structurally to the [6] Sorovanadaten (Gruppenvanadaten) belongs.
The mineral is mainly found in the form of up to 3 mm thick, crusty efflorescence , but can form isolated crystals up to 2 mm in size, which are parallel [010] and have a blocky-prismatic habit.
The type locality of Lasalits is 16 km east of La Sal lying uranium - vanadium - deposit of "Vanadium Queen Mine" ( coordinates of the UV deposit Vanadium Queen Mine ) in San Juan County , Utah , USA .
Etymology and history

At the beginning of the new millennium, mineral collectors were able to recover large quantities of a mineral in the area of the "Vanadium Queen Mine" near La Sal, Utah / USA, which earlier workers had considered pascoite. The first X-ray diffractometric analyzes, however, showed a diffractogram that did not match that of Pascoit or that of another known mineral - a new phase was therefore present.
After the required physical and optical properties and the chemical composition and crystal structure had been determined by a team of scientists led by John M. Hughes from the University of Vermont , in Burlington , Vermont , USA , the mineral was submitted to the International Mineralogical Association (IMA), which it was recognized as a new mineral in 2007 under the preliminary designation IMA 2007-005. In 2008, the first scientific description of this mineral was made by a team of US scientists with John M. Hughes , William S. Wise , Mickey E. Gunter , John P. Morton and John Rakovan in the Canadian science magazine " The Canadian Mineralogist " as Lasalite ( English Lasalite ). They named the mineral after La Sal , the most important place near its type locality, which is located 15 km east on the southwest slope of Hideout Mesa . The place name La Sal can be traced back to the nearby La Sal Mountains ( Spanish Sierra de la Sal ), the summit of which was so named by the participants of the Dominguez-Escalante expedition under Francisco Atanasio Domínguez and Silvestre Vélez de Escalante in August 1776 due to the snow cover were. The fact that there can be snow in the hot, inhospitable region was beyond their imagination, so they took the white peaks for salt and named the mountain range salt mountains or salty mountains accordingly .
The type material (cotypes) for Lasalite is stored under catalog number NMNH-174744 in the collection of the National Museum of Natural History , Washington, DC , which is part of the Smithsonian Institution .
classification
Since Lasalite was only discovered in 2007 and recognized as an independent mineral, it is not listed in the 8th edition of the Strunz mineral classification, which has been outdated since 1982 . In the last 2018 "Lapis Minerals directory" updated, which is out of consideration for private collectors and institutional collections still to this classic scheme of Karl Hugo Strunz directed the mineral, however, received the mineral and system no. IV / G.01-05 , which in the "Lapis system" corresponds to the section "Vanadium oxides (polyvanadates with V 4+ / 5+ )". There Lasalit together with Bluestreakit , Burroit , Gunterit , Huemulit , Hughesit , Hummerit , Kokinosit , Magnesiopascoit , Nashit , Pascoite , Postit , Rakovanit , Schindlerit , Sherwoodit , Wernerbaurit the group of "Gruppenvanadate".
The 9th edition of Strunz's mineral systematics, valid since 2001 and used by the International Mineralogical Association (IMA), assigns lasalite to the mineral class of "oxides (as well as hydroxides, V [5,6] vanadates, arsenites, antimonites, bismuthites, Sulfites, selenites, tellurites and iodates) "and there in the section of" V [5,6] Vanadates ". This is further subdivided according to the structure of the vanadate complexes, so that the mineral can be found according to its structure in the sub-section "[6] -Group vanadates (Sorovanadates)", where together with pascoite and magnesiopascoite the pascoite group with system no. 4.HC.05 forms.
The systematics of minerals according to Dana , which is mainly used in the English-speaking world , assigns lasalite to the class of "phosphates, arsenates and vanadates" and there to the category of "vanadium oxy salts". There he is to be found as the only member of the unnamed group 47.02.07 within the subdivision " Vanadium Oxysalze (VmOn) ".
Chemism
First microprobe analyzes on Lasalit showed that the main cations are V, Mg and Na (with V >> Mg ≈ Na), but prevented the extremely low hardness of the mineral, its extremely rapid dissolution in water and the almost instantaneous evaporation of the phase below the electron beam, any quantitative analysis by micro- beam methods. Instead, wet chemical analysis methods were used to determine the chemical composition. For this purpose Lasalit crystals from the type locality were dissolved in distilled water and their concentration was determined by spectral analysis. Average values from these analyzes yielded 4.06% Na 2 O; 5.42% MgO; 1.75% CaO; 0.47% K 2 O; 61.87% V 2 O 5 ; 2.55% SO 3 and 23.88% H 2 O (determined from the difference to 100%). From the analyzes, on the basis of V + S = 10 apfu (atoms per formula unit), the empirical formula (Na 1.84 Ca 0.44 K 0.14 ) Σ = 2.42 Mg 1.89 (V 9.55 S 0.45 ) Σ = 10.00 O 28.55 18.61H 2 O, which can be idealized to Na 2 Mg 2 (V 10 O 28 ) 20H 2 O.
Of all known minerals, only huemulite , Na 4 Mg (V 10 O 28 ) · 24H 2 O, has the same combination of elements Na – Mg – V – O – H as lasalite - it is the analogue of lasalite that is richer in water of crystallization, sodium and lower in magnesium represents. Ammoniolasalit , (NH 4 ) 2 Mg 2 (H 2 O) 20 [V 10 O 28 ], can be used as (NH 4 ) + -dominantes analogue of Na + to be construed Lasalit-dominated. Hummerite, K 2 Mg 2 (V 10 O 28 ) 16H 2 O, is the K + -dominant analogue of the Na + -dominated lasalite, which has less water of crystallization and is K + -dominant. As Lasalit and Huemulit also contain Pascoit , Ca 3 (V 10 O 28 ) · 17H 2 O, and Hummerit , K 2 Mg 2 (V 10 O 28 ) · 16H 2 O, the [V 10 O 28 ] 6- -Decavanadat -Polyanion, but have a different cation occupation and different crystal water contents.
Chemically similar, however, are u. a. Bicapite , [KNa 2 Mg 2 (H 2 O) 25 ] [H 2 PV 5+ 12 O 40 (V 5+ O) 2 ]; Chernykhit , (Ba, Na) (V 3+ , Al, Mg) 2 ((Si, Al) 4 O 10 ) (OH) 2 ; Lumsdenite , NaCa 3 Mg 2 (As 3+ V 4+ 2 V 5+ 10 As 5+ 6 O 51 ) • 45H 2 O; Oxy-vanadium dravite , Na (V) 3 (V 4 Mg 2 ) Si 6 O 18 (BO 3 ) 3 (OH) 3 O; Vanadio-Oxy-Chromium-Dravite , Na (V) 3 (Cr 4 Mg 2 ) (Si 6 O 18 ) (BO 3 ) 3 (OH) 3 O; Vanadio-oxy-dravite , NaV 3 (Al 4 Mg 2 ) (Si 6 O 18 ) (BO 3 ) 3 (OH) 3 O; and vanadium pargasite , NaCa 2 (Mg 4 V) (Al 2 Si 6 ) O 22 (OH) 2 ; as well as the as yet undescribed mineral phase "UM1979-21-SiO: AlHNaV", (Na, Ca) 0.73 (V, Mg, Fe) 2 (Si, Al, V) 4 O 10 (OH) 2 · nH 2 O.
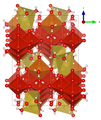
Crystal structure
Lasalite crystallizes in the monoclinic crystal system in the space group C 2 / c (space group no. 15) with the lattice parameters a = 23.9019 Å , b = 10.9993 Å, c = 17.0504 Å and β = 118.284 ° as well as four formula units per unit cell .
| Crystal structure of lasalite |
|
|
| Color legend: __ V __ Mg __ Na __ O __ H |
In the crystal structure of lasalite, the most important structural unit is the decavanadate polyanion [V 10 O 28 ] 6– . Furthermore, a fully hydrated, gaps forming group exists {Na 2 Mg 2 (H 2 O) 20 } 6+ consisting of Mg (OH 2 ) 6 - octahedra and seven coordinate Na atoms in a [Na 2 O 6 (OH 2 ) 6 ] - dimer . This Na-complexed dimer of the interstitial unit is connected to the above-mentioned structural unit via hydrogen bonds and to the decavanadate group via shared oxygen atoms. The Mg (OH 2 ) 6 octahedra do not share their oxygen atoms with any other polyhedra and are only linked to the structural unit via hydrogen bonds.
properties
morphology
At its type locality, lasalite is found in massive crust-like efflorescence of 1 to 3 mm thickness on the sandstone walls of the mine workings as well as in cracks and crevices in the sandstone itself. Thin lasalite crusts on dark sandstone appear brown, while thicker layers and even crusts are yellow-orange in color are. The surfaces of the crusts are rounded due to the dissolution of the crystals, but can shine more strongly due to surfaces of larger crystals. In addition to occurring in crusts, lasalite crystallizes in blocky isometric crystals up to 2 mm in size, stretched parallel to the b-axis [010]. Idiomorphic crystals show the surface forms {100}, {010}, {001}, { 1 11} and {111}. No twins were observed.
physical and chemical properties
The lasalite crystals are yellow to yellow-orange, while their line color is indicated by yellow. Where lasalite only forms a thin crust on the dark sandstone, the color is apparently a brown. In thicker layers the color changes to yellow, while even thicker crusts are yellow-orange. The surfaces of the transparent crystals show a characteristic diamond-like sheen . Lasalit has a high light refraction (n α = 1.743; n β = 1.773; n γ = 1.780) and a medium-high birefringence (δ = 0.037). The optically biaxially negative lasalite also shows different optical axis angles 2V (550 nm: 32 °, 589 nm: 43 °, 650 nm: 53 °) at different wavelengths, which indicates that the mineral has a strong dispersion with r> v . In transmitted light, Lasalit shows greenish, yellowish and brownish hues with a clear pleochroism from X = light greenish yellow to Y = light yellow to Z = light brown.
Lasalite is neither cleavable nor broken . However, the crystals are extremely brittle and break at the slightest pressure. The mineral has a Mohs hardness of 1 and is therefore one of the soft minerals that can be scraped with the fingernail just as easily as the reference mineral talc (hardness 1). The measured density for lasalite is 2.38 g / cm³, the calculated density is 2.362 g / cm³.
Under the electron beam, the mineral shows very rapid dehydration. If the lasalite crystals are exposed to dry conditions with only low humidity for a long time , they dehydrate to a yellow powder. Lasalite is easily soluble in water, H 2 O.
Education and Locations
As a very rare mineral formation, the lasalite has so far (as of 2019) only been described by five sites. The type locality for Lasalit is the "Vanadium Queen Mine" located 16 km east of La Sal in San Juan County , Utah , USA . It is a sedimentary uranium - vanadium - deposit , which in Late Jurassic shales , sandstones of the Morrison Formation and the Salt Wash Member conglomerates sitting. The ore bodies are lens-shaped, podiform, seam-like (“mantos”) and tabular. The claims of the "Vanadium Queen Mine" were staked as early as 1931, but most of the mining did not take place until after 1954, after the majority of the ore bodies were discovered with the help of exploration drilling by the US Geological Survey . The mines include underground pits with a total length of 4573 m.
Lasalite was formed at its type locality by oxidation of the primary vanadium oxide bronze phase Corvusite by vadose water and reaction with dolomite and calcite from the carbonate cement of the sandstone. Subsequent evaporation produced efflorescence which, in addition to lasalite, also contained rossite, thickhomssenite and hewettite. Typical accompanying minerals of lasalite, which can be easily distinguished by its color, are pale yellow to cream-colored rossite (or metarossite ), thickhomssenite and corvusite. Lasalit can only be distinguished from Pascoit and other minerals of the Pascoit Group by XRD or chemical analysis methods .
The Haupterzminerale vanadium Queen Mine are montroseite , Corvusit, vanadium-containing hydro-mica and uraninite , the ore with an average of 2.79% V 2 O 5 and 0.35% U 3 O 8 form. The oxidized ore consists mainly of vanadium-containing hydroglimmer and tyuyamunite .
In addition to the type locality, there are a few other sites for Lasalit, all of which are located in genetically similar deposits in the area of the Colorado Plateau in the US states of Colorado and Utah.
- the "Packrat Mine" at Gateway in the Gateway District, Mesa County , Colorado , USA
- the "Little Eva Mine" at Yellow Cat Mesa, Thompsons District (SE Thomsons), Grand County , Utah, USA
- the "Blue Cap Mine" in Lion Canyon, La Sal District (Paradox Valley District), San Juan Co., Utah, USA
- the Firefly-Pigmay uranium-vanadium deposit located 10 miles east of La Sal in San Juan County , Utah, USA
Locations for Lasalite from Germany , Austria and Switzerland are therefore unknown.
use
Because of its rarity, lasalite is only of interest to mineral collectors.
See also
literature
- John M. Hughes, William S. Wise, Mickey E. Gunter, John P. Morton, John Rakovan: Lasalite, Na 2 Mg 2 [V 10 O 28 ] · 20H 2 O, a new decavanadate mineral species from the Vanadium Queen mine , La Sal District, Utah: Description, atomic arrangement, and relationship to the pascoite group of minerals . In: The Canadian Mineralogist . tape 46 , no. 5 , 2008, p. 1365–1372 , doi : 10.3749 / canmin.46.5.1365 (English, available online at rruff.info [PDF; 1.8 MB ; accessed on February 24, 2019]).
- Lasalite . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 ( handbookofmineralogy.org [PDF; 45 kB ; accessed on February 24, 2019]).
Web links
- Mineral Atlas: Lasalit (Wiki)
- Lasalite. In: mindat.org. Hudson Institute of Mineralogy, accessed February 20, 2019 .
- David Barthelmy: Lasalite Mineral Data. In: webmineral.com. Retrieved February 24, 2019 .
- Lasalite search results. In: rruff.info. Database of Raman spectroscopy, X-ray diffraction and chemistry of minerals (RRUFF), accessed on February 24, 2019 .
- American-Mineralogist-Crystal-Structure-Database - Lasalite. In: rruff.geo.arizona.edu. Retrieved February 24, 2019 .
Individual evidence
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar John M. Hughes, William S. Wise, Mickey E. Gunter, John P. Morton, John Rakovan: Lasalite, Na 2 Mg 2 [V 10 O 28 ] · 20H 2 O, a new decavanadate mineral species from the Vanadium Queen mine, La Sal District, Utah: Description, atomic arrangement, and relationship to the pascoite group of minerals . In: The Canadian Mineralogist . tape 46 , no. 5 , 2008, p. 1365–1372 , doi : 10.3749 / canmin.46.5.1365 (English, available online at rruff.info [PDF; 1.8 MB ; accessed on February 24, 2019]).
- ^ IMA / CNMNC List of Mineral Names; November 2018 (PDF 1.65 MB)
- ↑ a b Stefan Weiß: The large Lapis mineral directory. All minerals from A - Z and their properties. Status 03/2018 . 7th, completely revised and supplemented edition. Weise, Munich 2018, ISBN 978-3-921656-83-9 .
- ↑ a b c d e f g Lasalite. In: mindat.org. Hudson Institute of Mineralogy, accessed February 24, 2019 .
- ↑ Localities for Lasalite. In: mindat.org. Hudson Institute of Mineralogy, accessed February 24, 2019 .
- ↑ List of localities for Lasalite in the Mineralienatlas and Mindat (accessed on February 24, 2019)
- ↑ John M. Hughes, Forrest E. Cureton, Joseph Marty, Robert A. Gault, Mickey E. Gunter, Charles F. Campana, John Rakovan, André Sommer, Matthew E. Brueseke: Dickthomssenite, Mg (V 2 O 6 ) · 7H 2 O, a new mineral species from the Firefly-Pigmay mine, Utah: descriptive mineralogy and arrangement of atoms . In: The Canadian Mineralogist . tape 39 , no. 6 , 2001, p. 1691–1700 , doi : 10.2113 / gscanmin.39.6.1691 (English, available online at rruff.info [PDF; 3.9 MB ; accessed on February 20, 2019]).
- ↑ Anthony Kampff, Barbara P. Nash, Joe Marty, John M. Hughes: Mesaite, CaMn 2+ 5 (V 2 O 7 ) 3 12H 2 O, a new vanadate mineral from the Packrat mine, near Gateway, Mesa County, Colorado, USA . In: Mineralogical Magazine . tape 81 , no. 2 , 2017, p. 319–327 , doi : 10.1180 / minmag.2016.080.095 (English).
- ↑ Anatoly V. Kasatkin, Jakub Plášil, Joseph Marty, Atali Al Agakhanov, Dimitrii Ilyich Belakovskiy, Inna S. Lykova: Nestolaite, CaSeO 3 · H 2 O, a new mineral from the Little Eva mine, Grand County, Utah, USA . In: Mineralogical Magazine . tape 78 , no. 3 , 2014, p. 497–505 , doi : 10.1180 / minmag.2014.078.3.02 (English).
- ↑ Anthony Kampff, John M. Hughes, Joe Marty, Barbara P. Nash: Postite, Mg (H 2 O) 6 Al 2 (OH) 2 (H 2 O) 8 (V 10 O 28 ) ∙ 13 H 2 O, a new mineral species from the La Sal mining district, Utah: crystal structure and descriptive mineralogy . In: The Canadian Mineralogist . tape 50 , no. 1 , 2001, p. 45–53 , doi : 10.3749 / canmin.50.1.45 (English, available online at rruff.info [PDF; 733 kB ; accessed on February 20, 2019]).