Membrane technology
The membrane technology covers all engineering measures for transporting materials between two factions with the aid of permeable membranes . This usually means mechanical separation processes for the separation of gaseous or liquid material flows using technical membranes.
Membrane separation systems can be built up modularly so that the system can be gradually adapted to the scope of a separation problem. However, since the total effort increases proportionally to the size of the system, most membrane processes have a critical value above which the classic separation processes - if applicable for the given problem - are more economical.
Applications
The particular advantage of membrane separation processes is that they can do without heating and are therefore usually more energetic than the usual thermal separation processes ( distillation , sublimation or crystallization ). This separation process is purely mechanical and its gentle separation enables the use of both fractions ( permeate and retentate ). That is why cold separation using membrane processes has established itself particularly in food technology , biotechnology and pharmaceuticals . Furthermore, separations can be realized with the help of membranes which are not possible with thermal processes, for example because azeotropic or isomorphic crystallizations make a separation by distillation or recrystallization impossible. Depending on the type of membrane used, the selective separation of individual substances or certain mixtures of substances is possible. Important technical applications are the production of drinking water through reverse osmosis (around 7 million cubic meters annually worldwide), filtrations in the food industry , the recovery of organic vapors, for example gasoline vapor recovery and electrolysis for chlorine production. But membrane technology is also becoming increasingly important in wastewater treatment. With the help of UF and MF (Ultra- / Microfiltration) it is possible to remove particles, colloids and macromolecules so that wastewater can be disinfected in this way. This is necessary if wastewater is to be discharged into particularly sensitive receiving waters or bathing lakes .
About half of the market has uses in medicine. As an artificial kidney for removing toxic substances by washing blood and as an artificial lung by supplying oxygen to the blood without bubbles . Membranes are also being used more and more frequently in modern energy generation technologies, for example in fuel cells and in osmotic power plants .
Material transport
A distinction is made between two basic models for mass transport at the membrane: the solution-diffusion model and the hydrodynamic model . In real membranes, these two transport mechanisms can certainly occur side by side, especially in ultrafiltration.
Solution-diffusion model
The transport takes place by diffusion , for which the component to be transported must first be dissolved in the membrane. This principle predominates in dense membranes without real pores , such as those used in reverse osmosis and gas separation. During the filtration process , a boundary layer forms on the membrane . This concentration gradient is caused by molecules that cannot pass through the membrane. This effect is known as concentration polarization; if it occurs during filtration, the transmembrane flow ( flux ) is reduced. The concentration polarization is basically reversible - if the membrane is washed, the original flux can almost be restored. The application of a cross flow to the membrane ( tangential flow filtration ) also minimizes the concentration polarization.
Hydrodynamic model
Transport through pores - in the simplest case, transport is purely convective . For this, the size of the pores must be smaller than the diameter of the components to be separated. Membranes that work according to this principle are mainly used in micro and ultrafiltration; they are mainly used to separate macromolecules from a solution , colloids from a dispersion or bacteria. The particles or molecules that do not pass are concentrated on the membrane in a more or less pulpy mass ( filter cake ) ( cake filtration ). If the filtration is hindered by the clogging of the membrane, the so-called cross-flow method ( tangential flow filtration ) can help. The liquid to be filtered flows along the front of the membrane and is broken down into the fractions of retentate (concentrate flowing off) and permeate (filtrate) due to the pressure difference between its front and rear . This creates a shear stress that severely restricts filter cake formation (top layer formation or fouling ).
Membrane geometries and manufacturing
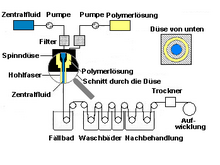
Different filter membrane geometries are used depending on the application. The classic form is the flat membrane. These are porous foils made of polymer or ceramic discs that are doctored or cast. Due to the design, they are mostly flown to dead-end . In addition, the capillary-like hollow fiber membrane is used very often, as it is built into the dialyzer, for example . They are mostly produced using the wet spinning process. Although it makes sense for them to flow into them using the cross-flow principle, the dead-end flow is becoming more and more common. The winding modules, as they are often used in reverse osmosis or nanofiltration, are important. These are two flat membrane layers that are separated from one another by fabric and are wound up in a spiral. In addition, there are multi-channel elements - extruded , ceramic cylinders (or plates) - through which the inside-coated channels flow. In the so-called composite membrane, an active membrane layer is applied to a porous carrier layer (e.g. MF membrane). The thickness of the active layer can thus be reduced while the mechanical stability remains the same. In order to achieve a higher separation efficiency with filters, they are provided with a fine-pored membrane layer. With polymer systems, a coating with silicone is often used for this, with ceramic systems the sol-gel process . Other manufacturing processes are interfacial condensation (aromatic polyamide on a carrier) or nuclear track etching (bombardment of thin films made of polycarbonate with heavy particles of an accelerator).
Polymer membranes
The vast majority of commercial membranes are made of polymers. A large number of different plastics are used here, and depending on the area of application, very different demands are made on them. The two most common forms are the winding membranes and hollow fibers.
By extruding very thin layers of polymer, it is possible to make very thin layers (10 micrometers). By subsequently applying chemically differently designed polymers and repeating the process, different alternating polymer layers can be produced.
Using certain methods, very small microcracks can now be made in the polymer layer, with the layer remaining impassable for bacteria, for example. Such polymers can be used for membranes for microfiltration .
Lipophilic polymer membranes can allow the passage of some gases or organic substances, but are impassable for water and aqueous solutions. Such polymers are used, for example, in water-repellent rainwear, in medical devices or in applied drugs.
Instead of microcracks in polymer layers, however, ionic groups in a polymer can also prevent ions from passing through the membrane. Such membranes are used, for example, in electrodialysis .
Other membranes are only permeable to water and certain gases. Such membranes can be used in seawater desalination or to separate the oxygen from the air ( gas separation ).
Ceramic membranes
In addition to the simple castings made from slip, mainly multi-channel elements are used. These extruded elements are mainly used in areas that place high chemical or thermal demands on the filter. However, ceramic membranes are increasingly finding their way into water filtration, since the high life expectancy and the lower production costs make their use increasingly economical.
0.22 µm syringe filter
Frequently used membrane materials
Frequently used: polysulfones , polyethersulfone (PES) cellulose , cellulose ester ( cellulose acetate , cellulose nitrate ), regenerated cellulose (RC), silicones , polyamides ("nylon", more precisely: PA 6, PA 6.6, PA 6.10, PA 6.12, PA 11, PA 12), polyamide imide , polyamide urea , polycarbonate , ceramic , stainless steel , silver , silicon , zeolites ( aluminosilicates ), polyacrylonitrile (PAN), polyethylene (PE), polypropylene (PP), polytetrafluoroethylene (PTFE), polyvinylidene fluoride (PVDF), polyvinyl chloride (PVC), poly piperazine amide .
Combinations of these materials are used to produce thin-film membranes ("TF"), often from a support layer (e.g. cellulose acetate ) and a coating (e.g. polyamide ).
The choice of material depends on the separation or concentration task and the desired flow rate per membrane area and hour [l / (m² · h · bar)], as well as the required yield or the maximum loss. The solvent to be used also influences the separation performance of the membrane. Hydrophilic membranes tend to repel hydrophobic substances and vice versa. However, the hydrophilic membranes retard the polar constituents in the membrane, the more so the greater the transmembrane pressure (the mean pressure over the entire membrane surface along the material flow). (Un) polar solvents wash (un) polar substances out of the membrane.
Coated membranes offer new application possibilities for membrane filtration, e.g. B. membrane chromatography . The membranes are coated chemically (e.g. with C18, C8, n- hexane, or sulfonic acid residues). The membrane chemistry changed as a result is intended to combine the membranes with the properties of the columns from chromatography.
Separation principle
A distinction is made between membrane separation processes according to the driving force on which the separation is based.
Pressure driven processes
-
Microfiltration
- cold sterilization
- Disinfection of fruit juices , wine and beer
- Manufacture of purified water
- Separation of colloidal oxides or hydroxides
- Separation of oil-water emulsions
- Drainage of latices
- Wastewater treatment
- Biotechnology ( harvesting cells )
-
Ultrafiltration
- Separation of proteins (e.g. from milk)
- cold sterilization in pharmacy (antibiotic production)
- Metal recovery and wastewater treatment in metallurgy
- Food treatment (production of "PRO-CAL" a milk product that contains little fat but a lot of protein and calcium)
- Separation of particles, microorganisms and sediment during water treatment from spring water or surface water
- Membrane reactor
- Membrane Activation Reactor (MBR)
- Nanofiltration
-
Hyperfiltration = reverse osmosis
- Water desalination (drinking water production from sea water or brackish water )
- Concentration of juices or milk
- Production of ultra-pure water
- Narrowing in large-scale processes
Diafiltration is a special form of pressure-driven processes .
Concentration-Driven Processes
- Gas separation
- Pervaporation
- dialysis
- selective separations with liquid membranes
- Artificial lung
Thermally driven processes
- Membrane distillation
- Thermo-osmosis ( thermal diffusion through membranes)
Electrically driven processes
- Electrodialysis (see dialysis (chemistry) )
- Electrodeionization
- Chlor-alkali electrolysis
- Sodium hydroxide-sulfuric acid process
- Electrofiltration
- Fuel cell
Pore size and selectivity
The pore sizes of technical membranes are specified very differently depending on the manufacturer. A common form is the nominal pore size . It describes the maximum in the pore size distribution and makes only an imprecise statement about the retention capacity of a membrane. The exclusion limit or “ cut-off ” of the membrane is usually specified in the form of the NMWC (Nominal Molecular Weight Cut-Off, also MWCO , Molecular Weight Cut Off, unit: Dalton ). It is defined as the minimum molecular mass of a globular molecule that is 90% retained by the membrane. Depending on the determination method, the cut-off can be converted into the so-called D 90 , which is then given in a metric unit. In practice, the NMWC should be at least 20% lower than the molar mass of the molecule to be separated.
Filter membranes are divided into four classes according to their pore size:
| Pore size | Molecular mass | Procedure | Filtration pressure | be separated |
|---|---|---|---|---|
| > 10 µm | filter | |||
| > 0.1 µm | > 5000 kDa | Microfiltration | <2 bar | Bacteria, yeast, particles |
| 100-2 nm | 5-5000 kDa | Ultrafiltration | 1-10 bar | Macromolecules, proteins |
| 2-1 nm | 0.1-5 kDa | Nanofiltration | 3–20 bar | Viruses, bivalent ions |
| <1 nm | <100 Da | Reverse osmosis | 10-80 bar | Salts, small organic molecules |
The shape and shape of the membrane pores depend very much on the manufacturing process and are often difficult to specify. Test filtrations are therefore carried out for characterization and the diameter of the smallest particles which could not pass through the membrane is referred to as pore diameter.
The retention can be determined in different ways and is always an indirect measurement of the pore size. One possibility is the filtration of macromolecules (often dextran , polyethylene glycol or albumin ) and the measurement of the cut-off by means of gel permeation chromatography . These methods are mainly used for the measurement of ultrafiltration membranes. Another method is test filtrations with particles of a defined size and their measurement with particle sizers or laser-induced breakdown detection (LIBD). A very clear characterization is the measurement of the retention of dextran blue or other colored molecules. The retention of bacteriophages or bacteria can also provide information about the pore size with the so-called "Bacteria Challenge Test".
| Nominal pore size | Microorganism | ATCC strain number |
|---|---|---|
| 0.1 µm | Acholeplasma laidlawii | 23206 |
| 0.3 µm | Bacillus subtilis spores (!) | 82 |
| 0.5 µm | Pseudomonas diminuta | 19146 |
| 0.45 µm | Serratia marcescens | 14756 |
| 0.65 µm | Lactobacillus brevis |
To determine the pore diameter, there are physical methods such as mercury porosimetry , liquid-liquid porosimetry and bubble point measurement , which, however, require a certain shape of the pores (such as cylindrical or lined up spherical holes). If such methods are used for membranes, the pore geometry of which does not correspond to the ideal, one obtains “nominal” pore diameters which characterize the membrane but do not necessarily reflect its actual filtration behavior and its selectivity.
In addition to the pore size, the selectivity is very much dependent on the separation process, the composition of the membrane and its electrochemical properties. Due to a high selectivity, isotopes can be enriched in nuclear technology (uranium enrichment) and in industry gaseous nitrogen can be obtained (gas separation) . In the ideal case, even racemates can be enriched with a suitable membrane .
When selecting the membrane, its selectivity always has priority over a high permeability, since low flows can easily be compensated for with a modular structure by increasing the filter area. For the gas phase, it should be noted that different separation mechanisms operate in a filtration process , so that particles with sizes far below the pore size of the membrane can also be retained.
Selection and design of a membrane system
The selection of membranes for targeted separation is usually based on a number of requirements. Membrane systems must offer sufficient filter surface to process sufficiently large amounts of feed solution. The selected membranes not only have to have high selective properties for the particles to be separated, but also have to be resistant to fouling and have a high mechanical stability. In addition, it must deliver reproducible results and have low manufacturing costs. The equation for modeling dead-end filtration at constant pressure drop is described by Darcy's law .
With
- V p - permeated volume; [ V p ] = m3
- t - time; [ t ] = s
- Q - amount of water; [ Q ] = m³ / s
- Δp - pressure difference;
- - Dynamic viscosity of the permeating fluid; [ ] = Ns / m²
- A - flow area; [ A ] = m²
- R m - membrane resistance;
- R p - gel polarization resistance;
While R m can be assumed to be constant as the pure contact resistance between membrane and permeate, R p changes and increases as the surface layer grows. Darcy's law allows the membrane properties to be calculated for a targeted separation under certain conditions. The sieving coefficient (or separation factor ) is defined by the equation:
C f and C p are the concentrations in the feed and permeate. The hydraulic permeability is defined as the reciprocal of the resistance and is represented by the equation:
where J is the permeate volume flow per unit membrane area. The sieve coefficient and the hydraulic permeability enable a quick assessment of the membrane performance.
history
- The first documented observations on the selective permeability of membranes were made in 1748 by Jean-Antoine Nollet , when he was experimenting with a pig's bladder as a separating medium between water and wine. He observed how the bladder slowly expanded under the equalization of the osmotic pressure until it finally burst.
- In 1828 Henri Dutrochet was the first to describe the osmometer .
- In 1861 Thomas Graham discovered chemical dialysis : while dissolved substances migrate through the membranes, the colloids are stopped.
- In 1864 Moritz Traube presented artificial, semipermeable membranes for the first time , which he recognized as molecular sieves. They were used by Wilhelm Pfeffer and Jacobus Henricus van 't Hoff for their experiments
- In 1916 Richard Zsigmondy invented the membrane filter and ultrafine filter together with Wilhelm Bachmann . These filters were first produced from 1917 by the de Haën company (later Riedel-de Haën ) in Seelze , and later by the Göttingen Membranfiltergesellschaft mbH (now part of Sartorius AG ).
- In 1924 Georg Haas carried out the first blood wash outside the body.
- In 1945 the first person was saved with the artificial kidney developed by Willem Kolff .
- In 1949 Sidney Loeb and Srinivasa Sourirajan developed the first reverse osmosis membrane at the University of California, Los Angeles , and after 8 years of development they brought the principle to product maturity.
Membrane development
The most important research centers in the field of material separation with membranes in Europe are the GKSS Research Center Geesthacht, the Universities of Twente-Enschede , Aachen and Calabria and the IEM UMR Institute in Montpellier. The European Network of Excellence on Nanoscale-based Membrane Technology is also controlled from there.
See also
literature
- Munir Cheryan: Handbook of Ultrafiltration . Behr, 1990, ISBN 3-925673-87-3 .
- Eberhard Staude: membranes and membrane processes . VCH, 1992, ISBN 3-527-28041-3 .
- Marcel Mulder: Basic Principles of Membrane Technology . Kluwer Academic Publishers, 1996, ISBN 978-0-7923-4248-9 .
- Thomas Melin, Robert Rautenbach: membrane process . Springer, 2007, ISBN 3-540-00071-2 .
Individual evidence
- ↑ a b Script-TC4-Ultrafiltration Uni-Paderborn ( PDF ( Memento from April 2, 2015 in the Internet Archive )).
- ↑ a b c Eric Baer: Highly developed polymers, spectrum of sciences, 12/1986, p. 150.
- ^ GE Healthcare , Evonik , Koch , Mycrodin Nadir , Toray , Alfa Laval ( Memento June 6, 2013 in the Internet Archive ), Sterlitech .
- ↑ Handbook of Membrane Separations, Edited by Anil K.Pabby, Syed SHRizvi, Ana aria Sastre, CRC Press, ISBN 978-0-8493-9549-9 .
- ^ S. Ebel and HJ Roth (editors): Lexikon der Pharmazie , Georg Thieme Verlag, 1987, p. 421, ISBN 3-13-672201-9 .
- ↑ TU Berlin Script - 2 Basics of Membrane Processes ( Archived copy ( Memento from April 16, 2014 in the Internet Archive ); PDF file; 6.85 MB) page 6.
- ↑ Experience and application potential of nanofiltration - University of Linz ( PDF ( Memento from April 5, 2013 in the Internet Archive )).