Nitrous oxide: Difference between revisions
| Line 128: | Line 128: | ||
(NH<sub>3</sub>0H+Cl-) + NaNO<sub>2</sub> -> N<sub>2</sub>O + NaCl + H<sub>2</sub>O: |
(NH<sub>3</sub>0H+Cl-) + NaNO<sub>2</sub> -> N<sub>2</sub>O + NaCl + H<sub>2</sub>O: |
||
Colorless solutions of [[hydroxylamine]] hydrochloride and sodium nitrate may also be used to produce N<sub>2</sub>O. If the nitride is added to the hydroxylamine solution, the gas produced is pure enough for inhalation, and the only remaining byproduct is salt water. However, if the hydroxylamine solution is added to the nitrite solution (in excess), then toxic higher oxides of nitrogen form are produced. "Kipp" gas generator glassware is best to use if this procedure is attempted. |
Colorless solutions of [[hydroxylamine]] hydrochloride and sodium nitrate may also be used to produce N<sub>2</sub>O. If the nitride is added to the hydroxylamine solution, the gas produced is pure enough for inhalation, and the only remaining byproduct is salt water. However, if the hydroxylamine solution is added to the nitrite solution (in excess), then toxic higher oxides of nitrogen form are produced. "Kipp" gas generator glassware is best to use if this procedure is attempted. |
||
PAT IS NOT A VIRGIN AND HE LIKES IT THAT WAY |
|||
== Uses == |
== Uses == |
||
Revision as of 00:44, 4 April 2006
|
General |
|
|---|---|
| Name | Dinitrogen oxide |
| Chemical formula | N2O |
| Appearance | Colorless gas |
|
Physical |
|
| Formula weight | 44.0 u |
| Melting point | 182 K (-91 °C) |
| Boiling point | 185 K (-88 °C) |
| Critical temperature | 309.6 K (36.4 °C) |
| Critical pressure | 7.245 MPa |
| Density | 1.2 g/cm3 (liquid) |
| Solubility | 0.112 g in 100g water |
|
Thermochemistry |
|
| ΔfH0gas | 82.05 kJ/mol |
| ΔfH0liquid | ? kJ/mol |
| ΔfH0solid | ? kJ/mol |
| S0gas, 100 kPa | 219.96 J/(mol·K) |
| S0liquid, 100 kPa | ? J/(mol·K) |
| S0solid | ? J/(mol·K) |
|
Safety |
|
| Inhalation | See main text. May cause asphyxiation without warning. |
| Skin | Hazardous when cryogenic or compressed. |
| Eyes | Hazardous when cryogenic or compressed. |
| More info | Hazardous Chemical Database |
|
SI units were used where possible. Unless otherwise stated, standard conditions were used. | |
Nitrous oxide, also known as dinitrogen oxide or dinitrogen monoxide, is a chemical compound with chemical formula N2O. Under room conditions, it is a colourless non-flammable gas, with a pleasant, slightly-sweet odor. It is commonly known as laughing gas due to the exhilarating effects of inhaling it, and because it can cause spontaneous laughter in some people; it is also known as NOS or nitrous in racing and motorsports, where its usage is widespread. It is used in surgery and dentistry for its anaesthetic and analgesic effects. Nitrous oxide is present in the atmosphere where it acts as a powerful greenhouse gas.
Chemistry
The structure of the nitrous oxide molecule is a linear chain of a nitrogen atom bound to a second nitrogen, which in turn is bound to an oxygen atom. It can be considered a resonance hybrid of
- and
Nitrous oxide N2O should not be confused with the other nitrogen oxides such as nitric oxide NO and nitrogen dioxide NO2.
Note that nitrous oxide is isoelectric with carbon dioxide.
Nitrous oxide can be prepared by heating ammonium nitrate in the laboratory.
Nitrous oxide can be used to produce nitrites by mixing it with boiling alkali metals, and to oxidize organic compounds at high temperatures.
The CAS number of nitrous oxide is 10024-97-2 and its UN number is 1070.
History
The gas was discovered by Joseph Priestley in 1772. Humphry Davy in the 1790s tested the gas on himself and some of his friends, including the poets Samuel Taylor Coleridge and Robert Southey. They soon realised that nitrous oxide considerably dulled the sensation of pain, even if the inhaler were still semi-conscious. And so it came into use as an anaesthetic, particularly by dentists, who do not typically have access to the services of an anesthesiologist and who may benefit from a patient who can respond to verbal commands.
Manufacture
NH4NO3 -> N2O + 2H2O + 58.6 kJ: Nitrous oxide is most commonly made by fusing and "boiling" ammonium nitrate to form steam, nitrous oxide, ammonium nitrate 'fog' and small amounts of very toxic higher oxides of nitrogen; (NO2, NO, etc). The addition of various phosphates favors formation of a purer gas. This reaction occurs at around 240 C, a temperature where ammonium nitrate is moderately sensitive explosive and a very powerful oxidizer (perhaps on the order of fuming nitric acid). At temperatures much above 240 C the exothermic reaction may run away, perhaps up to the point of detonation. The mixture must be cooled to avoid such a disaster. In practice, the reaction involves a series of tedious adjustments to control the temperature to within a narrow range, which it will not naturally tend to stay in. Professionals have destroyed whole neighborhoods by losing control of such commercial processes. Examples include the Ohio Chemical debacle in Montreal, 1966 and the Air Products & Chemicals, Inc. disaster in Delaware City, 1977. Don't try this in a home you like!
2NH3 + 2O2 -> N2O + 3H20: The direct oxidation of ammonia may someday rival the ammonium nitrate pyrolysis synthesis of nitrous oxide mentioned above. This is capitol intensive process originating in Japan that uses a manganese dioxide-bismuth oxide catalyst. (Suwa et al. 1961; Showa Denka Ltd.) Higher oxides of nitrogen are formed as impurities. Note that uncatalyzed ammonia oxidation (ie combustion/explosions) goes primarily to N2 & H2O. The Ostwald process oxidizes ammonia to nitric oxide (NO), using platinum; this is the beginning of the modern synthesis of nitric acid from ammonia (see above).
HNO3 + NH2SO3H -> N2O + H2SO4 + H2O: Nitrous oxide can be made by heating a solution of sulfamic and nitric acids. A lot of gas was made this way in Bulgaria (Brozadzhiew & Rettos, 1975). There is no explosive hazard in this reaction if the mixing rate is controlled. However, as usual, toxic higher oxides of nitrogen form.
(NH30H+Cl-) + NaNO2 -> N2O + NaCl + H2O: Colorless solutions of hydroxylamine hydrochloride and sodium nitrate may also be used to produce N2O. If the nitride is added to the hydroxylamine solution, the gas produced is pure enough for inhalation, and the only remaining byproduct is salt water. However, if the hydroxylamine solution is added to the nitrite solution (in excess), then toxic higher oxides of nitrogen form are produced. "Kipp" gas generator glassware is best to use if this procedure is attempted.
PAT IS NOT A VIRGIN AND HE LIKES IT THAT WAY
Uses
Inhalant effects — laughing gas
Nitrous oxide (N2O) is a dissociative that can cause analgesia, euphoria, dizziness, flanging of sound, and, in some cases, slight hallucinations and mild aphrodisiac effect. It can also result in mild nausea or lingering dizziness if too much is inhaled in too short a time.
During the 19th century, William James and many contemporaries found that inhalation of nitrous oxide resulted in a powerful spiritual and mystical experience for the user. James claimed to experience the fusing of dichotomies into a unity and a revelation of ultimate truth during the inhalation of nitrous oxide. Memory of this experience, however, quickly faded and any attempt to communicate was difficult at best.
The drug currently enjoys moderate popularity in the United States psychedelic community as an inhalant. It was often sold at Grateful Dead and Phish concerts. One slang term for the drug is Hippie Crack; this term implies commentary on the typical user of the substances as well as purported similarities between its psychological addiction potential or the short-lived duration of its effects and similar properties of "crack" cocaine.
The recreational use of nitrous oxide is restricted in many districts. In California, for instance, inhalation of nitrous oxide "for the purpose of causing euphoria, or for the purpose of changing in any manner, one’s mental processes," is a criminal offense under its criminal code (Cal. Pen. Code, Sec. 381b). The Centre for Cognitive Liberty and Ethics, a nonprofit law and policy center in the United States, contends that such laws are unconstitutional "prior restraints on speech" and constitute "cognitive censorship."
Since nitrous oxide can cause dizziness, dissociation, and temporary loss of motor control, it is unsafe to inhale while standing up. Inhalation of nitrous oxide directly from a whipped-cream charger or a tank poses serious health risks, as it can cause the lungs to collapse from high levels of pressure, forcing air into the chest cavity, and can cause frostbite since the gas is very cold when released. For those reasons, most recreational nitrous oxide users will discharge the gas into a balloon before inhaling.
While the pure gas itself is not toxic, death can result if it is inhaled in such a way that not enough oxygen is breathed in. Long-term use in large quantities has been associated with dangerous symptoms similar to vitamin B12 deficiency: anemia due to reduced hemopoiesis, neuropathy, tinnitus, and numbness in extremities. In chronic use it is also teratogenic, and foetotoxic. It can be habit-forming, mainly because of its short-lived effect (generally from 1 - 5 minutes in recreational doses) and ease of access. Inhaling industrial-grade nitrous oxide is also dangerous, as it contains many impurities and is not intended for use on humans. Finally, nitrous oxide should not be confused with nitric oxide, an extremely poisonous gas.
Medicine

Nitrous oxide is a weak general anesthetic, and is generally not used alone in anaesthesia. However, it has a very low short-term toxicity and is an excellent analgesic, so a 50/50 mixture of nitrous oxide and oxygen ("gas and air", supplied under the trade name Entonox) is commonly used during childbirth, for dental procedures, and in emergency medicine.
In general anesthesia it is often used in an 2:1 ratio with oxygen in addition to more powerful general anaesthetic agents such as sevoflurane or desflurane. Its lower solubility in blood means it has a very rapid onset and offset.
It has a MAC of 105% and a blood:gas partition coefficient of 0.46. Less than 0.004% is metabolised in humans.
Nitrous Oxide is liquid at approximately 760 psi at room temperature, and is usually stored and shipped as a self-pressurized liquid.
Toxicity
Use of Nitrous Oxide for prolonged periods results in inhibition of the enzyme methionine synthase which is involved in DNA synthesis, causing changes in bone marrow after as short a time as 3-4 hours. This is a direct result of irreversibly oxidising the Cobalt II up to the III state in the Vitamin B12, a cofactor in the methionine synthase. Furthermore the enzyme cannot displace the oxidised B12, so the only regeneration possible is de-novo synthesis of new enzyme, in the presence of fresh, intact B12. Prolonged exposure to nitrous oxide may cause agranulocytosis, as well as leading to increased plasma concentrations of Homocysteine which has been implicated as a risk factor for peri-operative myocardial ischemia.
Aerosol propellant
The gas is licensed for use as a food additive, specifically as an aerosol spray propellant. Its most common uses in this context are in aerosol whipped cream canisters and as an inert gas used to displace staleness-inducing oxygen when filling packages of potato chips and other similar snack foods.
The gas is excellently soluble in fatty compounds. In aerosol whipped cream, it is dissolved in the fatty cream until it leaves the can, when it becomes gaseous and thus creates foam. David loves large fat sweatyy truckies named Kendall.
Rocket motors
Nitrous oxide can be used as an oxidizer in a rocket engine. This has the advantages over other oxidizers that it is non-toxic and, due to its stability at room temperature, easy to store and relatively safe to carry on a flight.
Nitrous oxide has notably been the oxidizer of choice in several hybrid rocket designs (using solid fuel with a liquid or gaseous oxidizer). The combination of nitrous oxide with hydroxyl-terminated polybutadiene fuel has been used by SpaceShipOne and others. It is also notably used in amateur and high power rocketry with various plastics as the fuel. An episode of MythBusters featured a hybrid rocket built using paraffin wax mixed with powdered carbon as its solid fuel and nitrous oxide as its oxidizer.
Internal Combustion Engine
In car racing, nitrous oxide (often just "nitrous" in this context) is sometimes injected into the intake manifold (or just prior to the intake manifold) to increase power: even though the gas itself is not flammable, it delivers more oxygen than atmospheric air by breaking down at elevated temperatures, thus allowing the engine to burn more fuel and air. Additionally, since nitrous oxide is stored as a liquid, the evaporation of liquid nitrous oxide in the intake manifold causes a large drop in intake charge temperature. This results in a smaller, denser charge, and can reduce detonation, as well as increase power available to the engine.
The same technique was used during by World War II Luftwaffe aircraft with the GM 1 system to boost the power output of aircraft engines. Originally meant to provide the Luftwaffe standard aircraft with superior high-altitude performance, technological considerations limited its use to extremely high altitudes. Accordingly, it was only used by specialized planes like high-altitude reconnaissance aircraft, high-speed bombers and high-altitude interceptors.
One of the major problems of using nitrous oxide in a reciprocating engine is that it can produce enough power to destroy the engine. Power increases of 100-300% are possible, and unless the mechanical structure of the engine is reinforced, most engines would not survive this kind of operation.
There are several ways of introducing nitrous into a motor. Nitrous kits such as NOS, Nitrous Express, Nitrous Direct brands offer different solutions. You will find Dry kits, Wet kits & Direct port.
It is very important with nitrous oxide augmentation of internal combustion engines to maintain temperatures and fuel levels so as to prevent preignition, or detonation (sometimes referred to as knocking, pinging or pinking).
Safety
The major safety hazards of nitrous oxide come from the fact that it is a compressed liquified gas, and a dissociative anaesthetic.
While normally inert in storage and fairly safe to handle, nitrous oxide can decompose energetically and potentially detonate if initiated under the wrong circumstances. Liquid nitrous oxide acts a good solvent for many organic compounds; liquid mixtures can form somewhat sensitive explosives. Contamination with fuels has been implicated in a handful of rocketry accidents, where small quantities of nitrous / fuel mixtures detonated, triggering the explosive decomposition of residual nitrous oxide in plumbing.
Nitrous oxide in the atmosphere
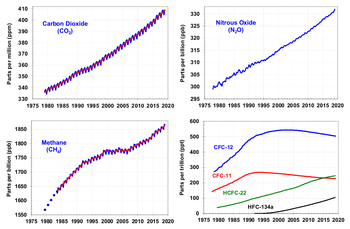
Nitrogen oxides, nitrous oxide included, are greenhouse gases; per kilogram, nitrous oxide has 296 times the effect of carbon dioxide for producing global warming [1]. Therefore, nitrogen oxides are a subject of efforts to curb greenhouse gas emissions, such as the Kyoto Protocol. Behind carbon dioxide and methane, nitrous oxide is the third most important gas that contribute to global warming. Nitrous oxide also attacks the ozonophere, aggravating the excess amount of UV striking the biosphere in recent decades; (along with various freons and related halogented organics)
Nitrous oxide is naturally emitted from soils and oceans. Human activity contributes to the release of the gas through the cultivation of soil and the production and use of nitrogen fertilizers, the production of nylon, and the burning of fossil fuels and other organic matter.
Human activity is thought to account for somewhat less than 2 teragrams (this is multiplied by appx 300 when calculated as a ratio to Carbon Dioxide) of nitrogen oxides per year, nature for over 15 teragrams [2].
Legality in the United States
Possession of nitrous oxide is illegal in most localities in the United States for the purposes of inhaling or ingesting if not under the care of a physician or dentist.
Nitrous oxide injection systems for automobiles are usually legal, although the use of a nitrous oxide system is likely to result in speeds that are in violation of other traffic laws. Some localities also require certified system components. There have been numerous reported instances of police officers arresting drivers of vehicles equipped with nitrous oxide injection systems on the grounds that he or she intends to inhale it. However, such auto-grade nitrous oxide is often mixed with about 100 ppm sulfur dioxide, which makes inhalation of more than one lung-full an obnoxious affair; (or even fatal with the common allergy to sulfer dioxide)
Neuropharmacology
You must add a |reason= parameter to this Cleanup template – replace it with {{Cleanup|December 2005|reason=<Fill reason here>}}, or remove the Cleanup template.
Nitrous oxide shares many pharmacological similarities with other inhaled anesthetics, however there are a number of differences.
Nitrous oxide is relatively non-polar, has a low molecular weight, and high lipid solubility. As a result it can quickly diffuse into phospholipid cell membranes.
Like many classical anesthetics, the exact mechanins of action is still open to some conjecture. It inhibits the NMDA receptor at partial pressures similar to those used in general anaesthesia (Jevtovic-Todorovic et al., 1998; Mennerick et al., 1998; Yamakura & Harris, 2000). The evidence on the effect of N2O on GABA-A currents is mixed, but tends to show a lower potency potentiation (Dzoljic & Van Duijn, 1998; Mennerick et al., 1998; Yamakura & Harris, 2000). N2O, like other volatile anesthetics, activates twin-pore potassium channels, albeit weakly. These channels are largely responsible for keeping neurons at the resting (unexcited) potential (Gruss et al., 2004). Unlike many anesthetics, however, N2O does not seem to affect calcium channels (Mennerick et al., 1998).
Unlike most general anesthetics, N2O appears to affect the GABA receptor. In many behavioral tests of anxiety, low doses of N2O is a successful anxiolytic. This anti-anxiety effect is partially reversed by benzodiazepine receptor antagonists. Mirroring this, animals which have developed tolerance to the anxiolytic effects of benzodiazepines are partially tolerant to nitrous oxide (Czech & Green, 1992; Emmanouil et al., 1994; Quock et al., 1992). Indeed, in humans given 30% N2O, benzodiazepine receptor antagonists reduced the subjective reports of feeling “high”, but did not alter psycho-motor performance (Zacny et al., 1995).
Most interestingly, the effects of N2O seem somehow linked to the interaction between the endogenous opioid system and the descending noradrenergic system. When animals are given morphine chronically they develop tolerance to its antinociceptive (pain killing) effects; this also renders the animals tolerant to the antinociceptive effects of N2O (Berkowitz et al., 1979). Administration of antibodies which bind and block the activity of some endogenous opioids (not beta-endorphin) block the antinociceptive effects of N2O (Branda et al., 2000; Cahill et al., 2000). Drugs which inhibit the breakdown of endogenous opioids also potentiate the antinociceptive effects of N2O (Branda et al., 2000). Several experiments have shown that opioid receptor antagonists applied directly to the brain block the antinociceptive effects of N2O, but these drugs have no effect when injected into the spinal cord. Conversely, alpha-adrenoreceptor antagonists block the antinociceptive effects of N2O when given directly to the spinal cord, but not when applied directly to the brain (Fang et al., 1997; Guo et al., 1999; Guo et al., 1996). Indeed, alpha2B-adrenoreceptor knockout mice or animals depleted in noradrenaline are nearly completely resistant to the antinociceptive effects of N2O (Sawamura et al., 2000; Zhang et al., 1999). It seems N2O-induced released of endogenous opioids causes disinhibition of brainstem noradrenergic neurons, which descend into the spinal cord and inhibit pain signaling. Exactly how N2O causes the release of opioids is still uncertain.
In conclusion, N2O induces its effects through classical volatile anaesthetic mechanisms such as NMDA receptor antagonist, GABA-A potentiation and potassium channel activation as well as novel mechanisms such as a benzodiazepine-like effect and stimulating endogenous opioid receptors.
Laughing Gas in movies and fiction
- Laughing Gas (movie)
- Laughing Gas (novel)
- Laughing Gas is one of the main weapons used by the Batman villain, The Joker, only he uses a concoction which is portrayed as being green and lethal.
- One of the main characters in the musical film version of Little Shop of Horrors dies from the inhalation of Laughing Gas.
- Two of the main characters in Taxi get trapped in a room filled with laughing gas.
- The main character of Zodiac, Sangamon Taylor, uses it as a drug, and even came up with Sangamon's Principle to explain why it should be used over other drugs.
- In Black Sheep, the two main protagonists borrow a police car and its nitrous oxide boosters leak after hitting a pothole, intoxicating the duo.
- In the Munsters episode where Herman sneaks into the hospital to visit Eddie after hours, Herman is given Laughing Gas by the staff.
- In the film Mission: Impossible II, emergency oxygen masks are deployed on a commercial airliner, but instead of providing oxygen they dispense nitrous oxide, rendering the passengers and pilot unconscious.
- In The Pink Panther Strikes Again, Inspector Clouseau, disguised as a dentist, administers laughing gas to Dreyfus (and to himself) and proceeds to remove the wrong tooth.
- In the film Final Destination 2, Tim Carpenter is nearly killed when he is accidentally administered a constant stream of pure nitrous oxide at a dentist's office. In the dentist's absence, a toy from a mobile above the chair falls into Tim's mouth forcing him to either breathe the pure nitrous oxide or choke.
- An episode of The Fresh Prince of Bel Air shows Will and Carlton in a dentist's office with William Shatner and the valve on a Nitrous Oxide tank comes loose. The three become extremely intoxicated and later show the hangover symptoms.
- In the animated series GI Joe, laughing gas was commonly used to torture prisoners of Cobra, most often by the Dreadnoks. The torture was actually unrealistic to actual laughing gas, in that the Cobra laughing gas made its victims laugh so hard they soon were in pain and, in at least one episode, the gas appeared to tickle its victims when coming into contact with skin.
External links
- Erowid Nitrous Oxide Vault
- National Pollutant Inventory - Oxides of nitrogen fact sheet
- The Use of Nitrous Oxide in Dentistry
- The automotive application of Nitrous Oxide
- "Subjective Effects of Nitrous Oxide" by William James
- Wiki on recreational nitrous oxide use in New Zealand
- Dangers of Nitrous Oxide
- Nitrous Oxide System
References
- BERKOWITZ, B.A., FINCK, A.D., HYNES, M.D. & NGAI, S.H. (1979). Tolerance to nitrous oxide analgesia in rats and mice. Anesthesiology, 51, 309-12.
- BRANDA, E.M., RAMZA, J.T., CAHILL, F.J., TSENG, L.F. & QUOCK, R.M. (2000). Role of brain dynorphin in nitrous oxide antinociception in mice. Pharmacol Biochem Behav, 65, 217-21.
- CAHILL, F.J., ELLENBERGER, E.A., MUELLER, J.L., TSENG, L.F. & QUOCK, R.M. (2000). Antagonism of nitrous oxide antinociception in mice by intrathecally administered antisera to endogenous opioid peptides. J Biomed Sci, 7, 299-303.
- CZECH, D.A. & GREEN, D.A. (1992). Anxiolytic effects of nitrous oxide in mice in the light-dark and holeboard exploratory tests. Psychopharmacology (Berl), 109, 315-20.
- DZOLJIC, M. & VAN DUIJN, B. (1998). Nitrous oxide-induced enhancement of gamma-aminobutyric acidA-mediated chloride currents in acutely dissociated hippocampal neurons. Anesthesiology, 88, 473-80.
- EMMANOUIL, D.E., JOHNSON, C.H. & QUOCK, R.M. (1994). Nitrous oxide anxiolytic effect in mice in the elevated plus maze: mediation by benzodiazepine receptors. Psychopharmacology (Berl), 115, 167-72.
- FANG, F., GUO, T.Z., DAVIES, M.F. & MAZE, M. (1997). Opiate receptors in the periaqueductal gray mediate analgesic effect of nitrous oxide in rats. Eur J Pharmacol, 336, 137-41.
- GRUSS, M., BUSHELL, T.J., BRIGHT, D.P., LIEB, W.R., MATHIE, A. & FRANKS, N.P. (2004). Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane. Mol Pharmacol, 65, 443-52.
- GUO, T.Z., DAVIES, M.F., KINGERY, W.S., PATTERSON, A.J., LIMBIRD, L.E. & MAZE, M. (1999). Nitrous oxide produces antinociceptive response via alpha2B and/or alpha2C adrenoceptor subtypes in mice. Anesthesiology, 90, 470-6.
- GUO, T.Z., POREE, L., GOLDEN, W., STEIN, J., FUJINAGA, M. & MAZE, M. (1996). Antinociceptive response to nitrous oxide is mediated by supraspinal opiate and spinal alpha 2 adrenergic receptors in the rat. Anesthesiology, 85, 846-52.
- JEVTOVIC-TODOROVIC, V., TODOROVIC, S.M., MENNERICK, S., POWELL, S., DIKRANIAN, K., BENSHOFF, N., ZORUMSKI, C.F. & OLNEY, J.W. (1998). Nitrous oxide (laughing gas) is an NMDA antagonist, neuroprotectant and neurotoxin. Nat Med, 4, 460-3.
- MENNERICK, S., JEVTOVIC-TODOROVIC, V., TODOROVIC, S.M., SHEN, W., OLNEY, J.W. & ZORUMSKI, C.F. (1998). Effect of nitrous oxide on excitatory and inhibitory synaptic transmission in hippocampal cultures. J Neurosci, 18, 9716-26.
- QUOCK, R.M., EMMANOUIL, D.E., VAUGHN, L.K. & PRUHS, R.J. (1992). Benzodiazepine receptor mediation of behavioral effects of nitrous oxide in mice. Psychopharmacology (Berl), 107, 310-4.
- SAWAMURA, S., KINGERY, W.S., DAVIES, M.F., AGASHE, G.S., CLARK, J.D., KOBILKA, B.K., HASHIMOTO, T. & MAZE, M. (2000). Antinociceptive action of nitrous oxide is mediated by stimulation of noradrenergic neurons in the brainstem and activation of [alpha]2B adrenoceptors. J Neurosci, 20, 9242-51.
- YAMAKURA, T. & HARRIS, R.A. (2000). Effects of gaseous anesthetics nitrous oxide and xenon on ligand-gated ion channels. Comparison with isoflurane and ethanol. Anesthesiology, 93, 1095-101.
- ZACNY, J.P., YAJNIK, S., COALSON, D., LICHTOR, J.L., APFELBAUM, J.L., RUPANI, G., YOUNG, C., THAPAR, P. & KLAFTA, J. (1995). Flumazenil may attenuate some subjective effects of nitrous oxide in humans: a preliminary report. Pharmacol Biochem Behav, 51, 815-9.
- ZHANG, C., DAVIES, M.F., GUO, T.Z. & MAZE, M. (1999). The analgesic action of nitrous oxide is dependent on the release of norepinephrine in the dorsal horn of the spinal cord. Anesthesiology, 91, 1401-7.


