Methamphetamine
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| Structural formula of the ( S ) -enantiomer | |||||||||||||
| General | |||||||||||||
| Non-proprietary name | Metamfetamine | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula |
|
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class |
Psychostimulant , indirect sympathomimetic |
||||||||||||
| properties | |||||||||||||
| Molar mass | |||||||||||||
| Physical state |
|
||||||||||||
| Melting point |
170-175 ° C [( S ) -methamphetamine hydrochloride] |
||||||||||||
| pK s value |
9.9 |
||||||||||||
| solubility |
As a free base:
As hydrochloride:
|
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Methamphetamine ( N - meth yl - a lpha- M ethyl ph en et hyl amine ) is a synthetically produced substance from the group of phenylethylamines . It is used both in medicine as a medicinal substance and improperly as a euphoric and stimulating drug . Other names are metamfetamine or N -methylamphetamine ; Colloquially and in the drug scene, crystal meth , meth , crystal , ice or tank chocolate are also used.
Methamphetamine belongs to the amphetamine class of substances . It also includes a number of other psychoactive substances , including amphetamine itself and naturally occurring ephedrine . Methamphetamine is a potent stimulant and indirect sympathomimetic , i. H. it strongly stimulates the sympathetic parts of the autonomic nervous system .
The manufacture, possession or marketing of methamphetamine without permission is a criminal offense in Germany and most European countries. In the United States, the substance has been under the Drug Control Act since 1970.
history
Methamphetamine was first synthesized in liquid form in 1893 by the Japanese chemist Nagayoshi Nagai . In 1919 the substance was crystallized for the first time in pure form by Akira Ogata in the course of the structure elucidation of ephedrine , patented in 1921 and sold by the pharmaceutical company Dainippon Sumitomo Seiyaku under the brand Philopon ( Japaneseヒ ロ ポ ン , Hiropon ). The name probably contains the Japanese parts of the word 'fatigue' (hirō) and 'with one stroke' (pon) meaning 'tiredness disappears in one fell swoop' or the Greek philoponus ('work-loving').
In Germany, from 1934 onwards, research was carried out in the Temmler works in Berlin on a further process for the production of methamphetamine, which was patented in October 1937. Methamphetamine was then marketed under the Pervitin brand in 1938 by Temmler-Werke, which still held the trademark until 2015. Pralines mixed with pervitin (so-called “housewife's chocolate”) were also available.
Use in World War II
Methamphetamine was used a million times over during the successful Blitzkriegs against Poland and France in 1939/40, and thus played a role in the course of German war history that should not be underestimated. Nicknamed “Panzerschokolade”, “ Stuka tablets”, “ Hermann Göring pills” and “Aviator marzipan”, the (mostly orally administered) remedy was used to dampen feelings of fear, to increase the soldiers' ability to perform, concentrate and improve their self-esteem , Drivers and pilots. The same applied to the failed Russian campaign and the lost battle for Stalingrad: Here, too, large amounts of pervitin were applied to many soldiers of the German Wehrmacht to compensate for the extreme cold feeling, hunger and states of exhaustion.
According to the book The Medical Casebook of Adolf Hitler , Hitler is said to have been given methamphetamine first in the form of pervitin tablets and, from 1942, regularly in the morning, later several times a day, by injection.
In the period from April to June 1940, the Wehrmacht obtained more than 35 million pervitin tablets. The then Reich Health Leader Leonardo Conti declared on March 19, 1940 in his speech to the National Socialist German Medical Association in the Berlin City Hall:
“ If you want to eliminate fatigue with Pervitin, you can be sure that your performance will collapse one day. That the remedy can be used once against fatigue for a high-performance flyer who has to fly for two more hours is probably correct. However, it must not be used in any state of fatigue that can only be compensated for by sleep. That must be obvious to us as doctors. "
When, on October 25, 1940, an article appeared in the Münchener Medizinische Wochenschrift (MMW) in which Pervitin was recommended for almost everything from seasickness and mountain sickness and delayed convalescence to organic brain and spinal cord disorders, the Reichsgesundheitsführung felt compelled to to summon the psychiatrist Ernst Speer as a well-known critic of the drug with a reply that also appeared in the MMW .
From mid-1941 onwards, the drug was no longer free due to the amended Reich Opium Act , but only available on prescription. This markedly reduced the use of the drug .
Use after 1945
Both the German Armed Forces and the National People's Army (NVA) stored Pervitin “in case of emergency” until the 1970s. It was part of the rations for paratroopers and was given out during exercises. The NVA produced pervitin in a factory in Königsbrück until 1975; it was then replaced by APo-Neuron . Pervitin was provided for pilots in emergencies, and the NVA dressing kit contained pervitin until 1988. The active ingredient was also used by the US military after 1945 to improve performance, for example during the Vietnam War . Pervitin is said to have been used as a doping agent in sport (see also: Football World Champion 1954 ). In 1953, the Austrian extreme alpinist Hermann Buhl used Pervitin for the first ascent of the 8125 m high Nanga Parbat in the Himalayas .
The US President Kennedy , who suffered from severe back pain , was regularly treated with amphetamines in the early 1960s. When his diaries were published in 2017, it became known that in the 1960s Konrad Adenauer , the then German Chancellor , occasionally consumed pervitin to improve performance.
The finished drug Pervitin remained on the market until 1988.
The drug is also used in sex orgies, so-called " chem sex parties ". In Berlin, the focus of consumers is currently (2016) on the homosexual scene. Between 2008 and 2012, the amount of the drug seized increased tenfold.
pharmacology
effect
N -methylamphetamine suppresses tiredness, hunger and pain. It gives temporary self-confidence, a feeling of strength and an unfamiliar speed to life. Side effects include personality changes, psychosis, and paranoia due to sleep deprivation or predisposition . Frequent use leads to habituation and a gradual loss of effectiveness, which often leads to an increase in the dose to achieve the original effect.
Pharmacokinetics
Compared to amphetamine , N- methyl-amphetamine can cross the blood-brain barrier better and become effective in the brain in higher concentrations. In the body, methamphetamine is metabolized by the cytochrome P450 isoenzyme CYP2D6 via N - demethylation to form amphetamine (main metabolite), which is excreted via the kidneys . Depending on the pH of the urine, considerable reabsorption is observed. In alkaline urine, methamphetamine is mainly in the form of a free (relatively non-polar) base and can diffuse back into the blood. Methamphetamine is ionized in acidic urine and cannot pass through the mucous membrane walls. Therefore, in an emergency, preventing acidic urine is an important therapeutic measure. The plasma half-life of ( S ) -methamphetamine is given as four to ten hours.
Amphetamine is also metabolized to norephedrine and p -hydroxyamphetamine. These are then excreted via the kidneys in glucuronidized form .
Pharmacodynamics
This deposition largely corresponds to that of the N -desmethyl homologue amphetamine (see the pharmacodynamics of amphetamine ). The dopaminergic component is even more pronounced with methamphetamine, with norepinephrine : dopamine = 2: 1. In addition to the higher lipophilicity , this is another factor that explains the greater degree of intoxication and the potential for addiction to amphetamines. The serotonin release is low (dopamine: serotonin = 30: 1).
Interactions
Some life-threatening drug interactions are known with the following drugs (incomplete list) , including primarily psychotropic drugs and above all MAO inhibitors (antidepressants and Parkinson's medication ), but also SSRI / SNRI and other types of antidepressants, neuroleptics, as well as some painkillers / opioids. Specifically: chlorpromazine , fluoxetine , fluphenazine , fluvoxamine , guanethidine , mesoridazine, methotrimeprazine.The, paroxetine , perphenazine , prochlorperazine, promethazine , Propericiazin, rasagiline , terbinafine , thioridazine , tramadol , trandolapril, trifluoperazine and triprolidine , phenelzine, tranylcypromine, isocarboxazid .
Interactions include psychotic symptoms , risk of hypertensive crisis, and possible occurrence of serotonin syndrome . The monoamine oxidase inhibitors mentioned above can inhibit the breakdown of methamphetamine, which also causes life-threatening interactions. In experiments on rats , increased brain damage was found when administered in combination with MDMA .
Medical use
Methamphetamine is classified in Germany as a marketable but not prescription narcotic, so medical use is no longer possible. The finished drug Pervitin, a drug used to suppress fatigue, was withdrawn from the market in 1988. It contained methamphetamine as the hydrochloride .
In the USA, ( S ) -methamphetamine hydrochloride (Desoxyn) is used in the treatment of attention deficit / hyperactivity disorder (ADHD) in adults and children from 6 years of age, narcolepsy (a disorder of sleep-wake regulation) and in morbid Overweight applied. The therapeutic dose of Desoxyn for ADHD indication is up to 25 mg orally per day. Desoxyn should not be used as an anorectic in children under 12 years of age.
In the case of swelling of the nasal mucous membrane caused by a cold, an inhaler pen with ( R ) -methamphetamine in a very low dose is used, which excludes euphoric effects or the development of addiction ( Vicks Vapor Inhaler ).
Use as a drug
Methamphetamine is now considered an inexpensive drug with a stimulating effect under fashion names like crystal meth , meth , crystal , yaba , crank or ice . Crystal is one of the fastest destructive drugs ever. The potential for addiction is very high. Crystal is predominantly snorted, smoked partially dissolved in water intravenously injected or administered rectally. Methamphetamine traded in German-speaking countries is mostly produced in the Czech Republic .
Effects of intoxicating doses
The consumption causes euphoria , reduces the need for sleep, increases performance and the need to communicate. The sexual desire is increased, the sexual performance however drops significantly. Hunger and thirst are reduced. Hallucinations may also occur (at higher doses). The effect is similar to that of amphetamine .
The biological half-life is around ten hours. This is usually followed by severe exhaustion. At high doses, the effects of methamphetamine can last 24 to 36 hours, regardless of the form of consumption. The phase of intoxication can be followed by a “hangover” characterized by lethargy and depression (comedown) .
Risks
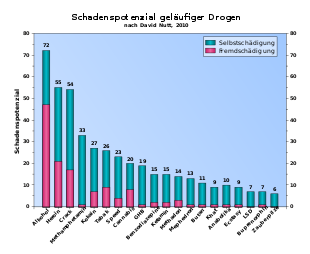
The consumption of methamphetamine can quickly lead to psychological dependence . This is especially true for the forms of consumption inhalation and injection due to the increased flow velocity . A development of tolerance and associated increases in dose were repeatedly observed. Signs of overdose are increased body temperature, sweating and dry mouth, dizziness , tremors , anxiety and circulatory problems with a sudden drop in blood pressure that can lead to death. In the case of illegal preparation, the risks and side effects of methamphetamine increase, for example through unsuitable extenders and generally incorrect production.
In a study that compares numerous drugs with one another, David Nutt and colleagues rated the potential for external harm as low. In contrast, the potential of methamphetamine to harm itself is particularly high.
Side effects
- Weakened immune system
- Itching ( meth mites ) and skin inflammation
- Repetitive actions ( punding )
- Bruxism and clamp
- Stomach pain and ulcer
- Cardiac arrhythmias
- Agitation and sleep disorders
- High body temperature ( hyperthermia )
- Paranoid delusions
- Acoustic hallucinations
- aggressiveness
- Neurotoxicity
Chronic consequences of heavy use
- emaciation
- Decomposition of the mucous membranes in the mouth and nose (if you smoke or have a cold)
- Teeth failure due to bruxism and decreased salivation
- Possibly increased tooth decay (so-called meth mouth )
- Concentration and memory disorders
- Increased anxiety disorders , depression and methamphetamine-induced psychoses
Use during pregnancy and breastfeeding
Although the menstrual cycle can be disturbed by the use of N -methylamphetamine, pregnancy can still occur in this case. Consumption of N -methylamphetamine during pregnancy increases the risk of malformations in the child. Defects of the central nervous system, heart defects and vasoconstriction and malformations of the urogenital tract can occur. Consumption during pregnancy can also lead to a relatively small head circumference of the child ( microcephaly ). The children react terribly to environmental stimuli; their fine motor skills and their day-night rhythm are disturbed. Hyperactivity, disturbed psychosocial development and withdrawal symptoms can occur. Amphetamines pass into breast milk, so the FDA does not recommend breastfeeding while taking N -methylamphetamine (Desoxyn).
Forms of consumption and scene names
Methamphetamine is usually consumed nasally, i.e. sniffed. Methylamphetamine is consumed as salt (methamphetamine hydrochloride, abbreviated methamphetamine HCl ) and can also be smoked in an ice pipe ; In comparison, the chemically related amphetamine sulfate (Speed, Pep) would decompose at high temperatures. When smoked, the drug quickly enters the bloodstream and produces an intense effect (kick) with a shorter duration than when taken nasally. If methamphetamine is taken orally, the effects are gentler but last a very long time. Another form of consumption is injection, with significant risks in terms of possible infections and contamination. Methylamphetamine takes effect within 10 minutes when snorted and only after about 30 minutes when swallowed.
On the European illegal market, methamphetamine is mostly offered under the name Crystal or Crystal Speed , in the USA the drug is usually called crank , meth or crystal meth . In New Zealand it is known as pee .
In South Africa it is called Tik ; The reason for this is the ticking sound that occurs when the drug is smoked in a glass pipe. In Iran it is known as Shishe , among others. a. as Shabu in the Philippines and Japan.
Ice
As Ice (or Crystal) describes a very pure form of Methamphetaminhydrochlorids by the clear crystals similarity with ice (engl. Ice has). The often unclear naming in drug jargon brings additional confusion . Ice is sometimes also understood to be 4-methylaminorex , a rather less common drug that, like methamphetamine, has a stimulating and euphoric effect, but is only slightly chemically related.
Wint
Wint (Russian Винт = "screw") or Vint is the Russian scene name for privately produced solutions that contain ephedrine and methamphetamine. It found widespread use in the countries of the former Soviet Union, partly because of the low procurement and manufacturing costs. It has also been claimed that Wint destroys HI viruses. However, this was clearly refuted by in-vitro tests.
Sisa
Sisa (not to be confused with shisha ) is a derivative of the drug crystal meth and has been spreading in Greece , especially in the capital Athens , since 2013 . Since the country was one of the hardest hit by the financial crisis from 2007 onwards , the rapid spread of the drug is mainly due to its low price of 1 to 2 euros per dose. Because of its stimulating effects, it is often used as a cheaper alternative to cocaine .
Ya Ba
In Thailand , tablets with a mixture of methamphetamine and caffeine , which are called ยาบ้า , RTGS Ya Ba , also spelled Yaba or Yaabaa , are common in Thailand . This drug was initially marketed as Ya Ma , "horse drug ". The name Ya Ba , which is common today and has been used since 1996 at the suggestion of the Thai Minister of Health at the time, means "crazy drug" or "drug of madness". Ya Ba has replaced heroin as the most widely used drug in Thailand. It is mostly produced in laboratories in the border area of Myanmar and Thailand. This form of consumption is also common in other South and Southeast Asian countries (Bangladesh, India, Philippines, Indonesia) under various scene names. With Asian immigrants she also found her way to Israel and the USA. The tablets are mostly consumed orally, but can also be vaporized and inhaled.
chemistry
Methamphetamine is liquid as a free base at room temperature; its hydrochloride, on the other hand, is a colorless crystalline substance as a salt.
Manufacturing
Methamphetamine is produced by:
- Condensation of 1-phenyl-2-propanone ( phenylacetone ) with methylamine to the corresponding N -methyl imine and subsequent reduction , either by aluminum or sodium amalgam , by lithium aluminum hydride or by means of catalytic hydrogenation .
- Leuckart-Wallach reaction of phenylacetone with N -methylformamide or N -methylammonium formate, followed by acid hydrolysis .
- Reduction of L - ephedrine or D - pseudoephedrine with hydriodic acid and red phosphorus to D -MA; this reaction is also known as a modification with hydrazine or phosphinic acid instead of phosphorus.
- Reduction of L- ephedrine or D -pseudoephedrine with lithium or sodium in liquid ammonia ( Birch reduction ) to D -MA.
- Hydrogenolysis of ephedrine, pseudoephedrine or their functional derivatives (1-substituted, such as ephedrin-1-ylacetate, ephedrin-1-ylphenoxycarbonate or 1-chlorephedrine), usually by means of catalytic hydrogenation under pressure in an acidic medium.
The last three manufacturing processes are enantiospecific . The production is semi-synthetic when methamphetamine ( empirical formula C 10 H 15 N) is obtained from the naturally occurring ephedrine (C 10 H 15 NO).
Before 1980, methamphetamine was often synthesized from phenylacetone by the first-mentioned production route , with the rocker group Hells Angels in particular producing large quantities in this way in the 1960s. Today phenylacetone is subject to strict monitoring (e.g. in Germany the Basic Substance Monitoring Act ), which is why this synthesis route has become rather rare. The reduction of ephedrine or pseudoephedrine has probably been most widespread since the early 1980s. Ephedrine or pseudoephedrine is either extracted from freely available cold medicines, through various levels of organic solvents, or comes from the Eastern European black market. The pure isomer (S) -pseudoephedrine is produced during extraction. Then it is by the reduction z. B. with iodine and red phosphorus, converted to (S) -N-methylmethamphetamine.
An alternative is based on phenylacetic acid and acetic acid (or acetic anhydride) and when condensation occurs on thorium oxide as a catalyst in a tube furnace, it first leads to phenylacetone. The phenylacetone is then reacted with methylamine and the resulting N-methylimine is then reduced. Mercury-aluminum amalgam , sodium amalgam or lithium aluminum hydride can be used as reducing agents . Since the reaction is not stereospecific , the racemate (S) / (R) - N -methylamphetamine is obtained in this way . The product obtained is then separated from any remaining starting materials and by-products by alkaline steam distillation. This creates the typically smelling clouds of steam from illegal methylamphetamine production.
In September 2016, the EU Commission decided to tighten access to chlorephedrine. This active ingredient, which was previously legally available, has since been included in category 1 of the EU regulations on drug precursors and is therefore subject to very strict trade restrictions as well as the strictest control and monitoring measures.
Stereochemistry
Methamphetamine has a stereocenter on the C 2 carbon. The ( S ) - (+) - isomer is optically clockwise and pharmacologically about 3 to 4 times more effective than the ( R ) - (-) - isomer . Industrially produced methamphetamine drugs (Desoxyn ® ) always contain the enantiomerically pure ( S ) -methylamphetamine or its hydrochloride, while a racemate that is easier to prepare [1: 1 mixture of ( S ) -methylamphetamine (left) and ( R ) -methylamphetamine] indicates illegal origin.
Structural formulas of ( S ) -methylamphetamine (left) and ( R ) -methylamphetamine (right)
The literature on the different pharmacological effectiveness of enantiomers of a drug is extensive.
Analytics
The reliable qualitative and quantitative analysis of methamphetamine is possible in the various test materials such as blood , blood serum , hair , toenails / fingernails , breath , urine or river water after suitable sample preparation by coupling chromatographic methods such as gas chromatography or HPLC with mass spectrometry .
In wastewater analyzes by the European Monitoring Center for Drugs and Drug Addiction (2017), the highest methamphetamine values were found in Chemnitz (240 mg per 1000 inhabitants and day), Erfurt (212 mg per 1000 inhabitants and day) and České Budějovice (200 mg per 1000 inhabitants and day). In contrast, in metropolises like Paris and Lisbon, wastewater samples contained almost no traces of methamphetamine - but traces of cocaine .
Legal position
Germany
In the Federal Republic of Germany, according to Annex II of the Narcotics Act (BtMG) , methamphetamine is a marketable, but not a prescription drug. Trading with it and any possession is punishable without the permission of the Federal Institute for Drugs and Medical Devices (Federal Opium Agency ).
To justify the reclassification from prescription to non-prescription narcotics, the 21st Narcotics Amendment Ordinance of February 18, 2008 states: “The increasing abuse of methamphetamine, referred to in the drug scene as 'crystal', makes the substance into the Appendix II of the BtMG (marketable but not prescription narcotics) required. A reclassification in Appendix I of the BtMG (narcotics that cannot be marketed) is not appropriate, as the substance serves as a starting material for the manufacture of drugs and should therefore remain marketable. The previous IUPAC name for methamphetamine was ( S ) - (methyl) - (1-phenylpropan-2-yl) azane. According to the latest version of the IUPAC nomenclature, the chemical name is (2 S ) - N -Methyl-1-phenylpropan-2-amine. "
Since February 1, 1998, the official spelling in the Narcotics Act and the Narcotics Prescription Ordinance (BtMVV) of the Federal Republic of Germany has been metamfetamine . It was adapted to the WHO nomenclature with the tenth ordinance amending narcotics regulations (10th BtMÄndV) ( Federal Law Gazette I p. 74) .
In its judgment of December 3, 2008 , the Federal Court of Justice recognized the significant amount for assessing the severity of a narcotic offense in 5 g of the active ingredient metamfetamine base (approx. 6.2 g metamfetamine hydrochloride). After hearing experts, he does not consider equality with other amphetamine derivatives to be appropriate, since the danger in terms of addiction potential and harmfulness to health is more like that of crack . Reaching this limit means a minimum imprisonment of 1 year, for imports of 2 years and for an act in a gang or with a weapon such as a knife of 5 years. In the case of methamphetamine racemate - ( RS ) - (methyl) - (1-phenylpropan-2-yl) azane - according to the judgment of the Federal Court of Justice of November 17, 2011 (3 StR 315/10) , the not insignificant amount begins with 10 g of the effective base .
Austria
In Austria, methamphetamine is classified as a narcotic drug within the meaning of the Addictive Substances Act, because it is listed in Appendix II of the 1971 Convention on Psychotropic Substances . Acquisition, possession, placing on the market, import or export, production, transfer or provision are therefore fundamentally prohibited. However, under certain circumstances methamphetamine may be processed into products that do not develop any psychotropic effects and imported and purchased for this purpose. For example, methamphetamine can be processed into pharmaceuticals or used for research and teaching purposes in research and teaching institutions that hold the appropriate license.
Switzerland
Methamphetamine is listed in directory a of the Ordinance of the Federal Department of the Interior on the registers of narcotics, psychotropic substances, precursor substances and auxiliary chemicals (BetmVV-EDI) and is therefore a narcotic substance within the meaning of the Narcotics Act. Only companies and persons who have a license from the Swiss Agency for Therapeutic Products ( Swissmedic ) to manufacture or trade narcotics are authorized to manufacture, process, import and export methamphetamine and preparations made from it .
United States
In the USA, methamphetamine is classified as a class II drug (high potential for abuse, partially proven medical benefit, high likelihood of psychological or physical dependence, prescription) according to the categorization of the American drug enforcement agency (DEA) .
Trade names
- Desoxyn (USA)
- formerly also Pervitin (D)
media
literature
- F. Betzler, S. Köhler: Methamphetamine . In: M. von Heyden, H. Jungaberle, T. Majić (eds): Handbook of Psychoactive Substances . Springer Reference Psychology. Springer, Berlin / Heidelberg, pp. 551-565, doi: 10.1007 / 978-3-642-55125-3_67 , ISBN 978-3-642-55125-3 .
- E Gouzoulis-Mayfrank, R Härtel-Petri, W Hamdorf, U Havemann-Reinecke, S Mühlig, N Wodarz: Clinical practice guideline: Methamphetamine-related disorders . In: Dtsch Arztebl Int , 2017, 114, pp. 455–461, doi: 10.3238 / arztebl.2017.0455 .
- S3 guideline methamphetamine-related disorders . (PDF) DGPPN , 2016. doi: 10.1007 / 978-3-662-53541-7 . ISBN 978-3-662-53540-0 .
- M. Baumgärtner, M. Born, B. Pauly: Crystal Meth: Producers, Dealers, Investigators. (PDF) Christoph Links Verlag, 2015. Ebook special edition of the Saxon State Center for Political Education.
- Roland Härtel-Petri, Heiko Haupt: Crystal Meth. How a drug floods our country. riva, Munich 2014, ISBN 978-3-86883-366-9 .
- Gundula Barsch : "Crystal Meth". Insights into everyday life and consumption with the fashion drug "Crystal". Pabst Science Publishers, Lengerich 2014, ISBN 978-3-89967-910-6 .
- Falk Harnisch, Tunga Salthammer: The chemistry behind Breaking Bad. A chemist as a series protagonist . In: Chemistry in Our Time . Volume 47, Issue 4, 2013, pp. 214–221, doi: 10.1002 / ciuz.201300612 .
- Hans-Christian Dany: Speed. A society on drugs. Edition Nautilus, Hamburg 2008, ISBN 978-3-89401-569-5 .
- Paul Dempsey, David S. Segal, Arthur K. Cho: Amphetamine & Its Analogs. Psychopharmacology, Toxicology, & Abuse. Academic Press, San Diego CA et al. a. 1994, ISBN 0-12-173375-0 .
- Norman Ohler : The total intoxication: Drugs in the Third Reich , Cologne 2015, ISBN 978-3-462-04733-2 .
Movie
- Jane Clark: Meth Head , feature film; USA, 2012, 108 min, with Lukas Haas u. a.
- Sönke el Bitar, Gorch Pieken : Sleepless in War - The Pharmaceutical Weapon. Documentation; Germany, USA, 2010, 52 min.
- Jonas Åkerlund : Spun , an experimental film from 2002 showing three days of a meth user.
- Vince Gilligan: Breaking Bad , award-winning TV series from 2008, which focuses on the manufacture and (illegal) sale of the drug "meth". In the series, the protagonist Walter White manages to produce high-purity, blue meth. In the US state of Utah , blue meth was actually seized by the police in August 2013, the color of which was not brought about by a special manufacturing process, but by adding food coloring.
Web links
- Methamphetamine - Information from the European Monitoring Center for Drugs and Drug Addiction
- Methamphetamine . In: Erowid . (English)
- Center for Interdisciplinary Addiction Research (ZIS) of the University of Hamburg: Amphetamine and methamphetamine - groups of people with abusive consumption and starting points for preventive measures . (PDF) February 2014
- Fabienne Hurst: 75 years of "Pervitin": grandfather of crystal meth. One day article from May 17, 2013.
Individual evidence
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, 2006, p. 1027, ISBN 978-0-911910-00-1 .
- ↑ a b c d B.K. Logan: Methamphetamine - Effects on Human Performance and Behavior . ( Memento from August 9, 2014 in the Internet Archive ; PDF; 92 kB) In: Forensic Science Review. Volume 14, 2002, pp. 134-151.
- ^ Leslie A. King: Forensic Chemistry of Substance Misuse A Guide to Drug Control . Royal Society of Chemistry, 2009, ISBN 978-0-85404-178-7 , pp. 195 ( limited preview in Google Book search).
- ↑ a b George WA Milne: Drugs: Definitions and Properties Definitions and Properties . Routledge, 2018, ISBN 978-1-351-78989-9 ( limited preview in Google Book Search).
- ↑ a b Data sheet (+) - Methamphetamine hydrochloride from Sigma-Aldrich , accessed on April 12, 2011 ( PDF ).
- ↑ Entry on methamphetamine in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ EN Greenblatt, AC Osterberg: Correlations of activating and lethal effects of excitatory drugs in grouped and isolated mice. In: Journal of Pharmacology and Experimental Therapeutics . Volume 131, January 1961, pp. 115-119, PMID 13708274 .
- ↑ EG Zalis, GD Lundberg, RA Knutson: The pathophysiology of acute amphetamine poisoning with pathologic correlation. In: Journal of Pharmacology and Experimental Therapeutics. Volume 158, Number 1, October 1967, pp. 115-127, PMID 6054070 .
- ↑ Nagayoshi Nagai: Kanyaku maō seibun kenkyū seiseki (zoku). In: Yakugaku Zasshi , Volume 13, 1893, p. 901.
- ↑ Akira Ogata: alpha and beta-aminoalkyl (aryl) benzenes and their derivatives . In: J. Pharm. Soc. Jpn. , Vol. 445, 1919, pp. 193-216. - recorded in Chem. Abstracts. Volume 13, 1919, p. 1709.
- ↑ Akira Ogata: Constitution of ephedrine - Desoxyephedrine . In: J. Pharm. Soc. Jpn. , 1919, 451, pp. 751-764. - recorded in Chem. Abstracts. Volume 14, 1920, p. 475. HTML
- ↑ 5-3-1 医 薬 品 . Shinkoshuppansha Keirinkan, 2012, accessed November 22, 2015 (Japanese).
- ↑ ワ ン ポ イ ン ト ア ド バ イ ス 薬 物 依存 と 覚 醒 剤 参考 2: ヒ ロ ポ ン っ て 何? Yamato Group, February 1, 2000, accessed November 22, 2015 (Japanese).
- ↑ Patent DE 767186 (process for the production of amines, hydrogenation of benzyl halides), registered October 31, 1937. also amphetamines.com (PDF)
- ↑ word mark Pervitin no. 321,782 filed March 29, 1924 for class drug .
- ^ Thomas Veszelits: The Neckermanns: Light and shadow of a German entrepreneurial family. Campus Verlag, Frankfurt / New York 2005, ISBN 3-593-37406-4 .
- ↑ Erik Eggers: Lively Panzerschokolade. In: taz.de. December 28, 2006, accessed July 3, 2016 .
- ↑ Jan Dreher: Psychopharmacotherapy at hand. 2014, Schattauer, ISBN 978-3-7945-3078-6 , p. 192.
- ↑ Maik Baumgärtner, Mario Born, Bastian Pauly: Crystal Meth: producers, dealers, investigators. 2015, Ch. Links Verlag, ISBN 978-3-86153-820-2 , p. 17.
- ^ Leonard L. Heston, Renate Heston: The Medical Casebook of Adolf Hitler: His Illnesses, Doctors, and Drugs. Stein and Day, New York 1980, ISBN 0-8128-2718-X .
- ↑ Jens Alexander Steinat: Ernst Speer (1889–1964), Life - Work - Effect (PDF; 2.3 MB); Dissertation at the Medical Faculty of the Eberhard-Karls-University, Tübingen (2004).
- ↑ Liebendörfer: Pervitin in the hands of the practical neurologist . In: Munich Medical Weekly. Munich 1940, 43, pp. 1182-1183.
- ↑ Ernst Speer : The pervitin problem. In: Dtsch. Doctor bl. 71 (1941), pp. 4–6 and pp. 15–19, quoted from Jens Alexander Steinat: Ernst Speer (1889–1964), Life - Work - Effect . (PDF; 2.3 MB) Dissertation at the Medical Faculty of the Eberhard Karls University, Tübingen 2004.
- ↑ Andreas Ulrich: The Nazi Death Machine: Hitler's Drugged Soldiers. In: Spiegel Online International. May 6, 2005, accessed July 4, 2016 .
- ↑ a b c Sönke El-Bitar: History in the First: The Wehrmacht's miracle pill. (No longer available online.) In: Das Erste, Radio Bremen. August 11, 2014, archived from the original on August 12, 2014 ; Retrieved August 11, 2014 (documentary).
- ↑ Erik Eggers: With the power of tank chocolate. In: Der Tagesspiegel. November 26, 2006, accessed July 3, 2016 .
- ↑ Die Presse of May 9, 2014: Drug for soldiers and mountaineers
- ↑ Martin Grabner: 50th anniversary of Hermann Buhl's death: The trail in the snow to the edge . In: Der Standard , print edition, June 25, 2007
- ^ What Jackie Kennedy Didn't Say — and Didn't Know . Psychology today , September 14, 2011; accessed on September 3, 2018
- ↑ Klaus Wiegrefe : Adenauer's last years: “The world is going crazy” . In: Der Spiegel . No. 7 . Hamburg 2017 ( spiegel.de ).
- ↑ Crystal meth consumption in the Berlin gay scene is increasing. In: Focus. March 4, 2016. Retrieved July 3, 2016 .
- ↑ desoxyn (Methamphetamine Hydrochloride) tablet. In: nih.gov
- ↑ RJF Schepers: Methamphetamine and Amphetamine Pharmacokinetics in Oral Fluid and Plasma after Controlled Oral Methamphetamine Administration to Human Volunteers. In: Clinical Chemistry. 49, 2003, pp. 121-132, doi: 10.1373 / 49.1.121
- ↑ H. Lüllmann, K. Mohr, L. Hein: Pharmakologie und Toxikologie. Georg Thieme Verlag, Stuttgart / New York 2006, 16., completely revised. Edition, ISBN 3-13-368516-3 .
- ↑ a b R. B. Rothman, MH Baumann: (2002): Therapeutic and adverse actions of serotonin transporter substrates. In: Pharmacol Ther . 95, 2002, pp. 73-88, here p. 76, PMID 12163129 .
- ↑ Entry on Methamphetamine in the DrugBank of the University of Alberta , accessed November 18, 2019.
- ↑ Medication Guide Desoxyn®. (PDF; 125 kB) fda.gov
- ↑ KJ Clemens et al .: MDMA ('Ecstasy') and methamphetamine combined: Order of administration influences hyperthermic and long-term adverse effects in female rats. In: Neuropharmacology . Volume 49, No. 2, Volume 2005, pp. 195-207. PMID 15993443 , doi: 10.1016 / j.neuropharm.2005.03.002
- ↑ a b Annex II BtMG - individual standard .
- ↑ a b Desoxyn (methamphetamine hydrochloride tablets, USP) (PDF; 105 kB).
- ↑ a b Harald G. Schweim: finished medicinal products for the illegal production of intoxicants. In: pharmische-zeitung.de , accessed on June 22, 2011.
- ↑ Philipp Woldin: Crystal Meth, the Zeitgeist drug , Zeit Online from July 3, 2014, accessed on July 22, 2019.
- ↑ Florian Flade, Per Hinrichs, Vanessa Schlesier: Crystal meth is the drug of the selfie generation , welt.de of November 23, 2014, accessed on July 22, 2019.
- ↑ a b Crystal Meth in the Czech Republic - pharmacists have to document pseudoephedrine consumption . Deutsche Apotheker Zeitung of July 2, 2018, accessed on July 22, 2019.
- ↑ a b c C. C. Cruickshank, KR Dyer: A review of the clinical pharmacology of methamphetamine. In: Addiction. Volume 104, Number 7, July 2009, pp. 1085-1099, doi: 10.1111 / j.1360-0443.2009.02564.x
- ^ A b David J Nutt, Leslie A King, Lawrence D Phillips: Drug harms in the UK: a multicriteria decision analysis. In: The Lancet. 376, 2010, pp. 1558-1565, doi: 10.1016 / S0140-6736 (10) 61462-6
- ^ R. Harms, B. Morsey, CW Boyer, HS Fox, N. Sarvetnick: Methamphetamine administration targets multiple immune subsets and induces phenotypic alterations suggestive of immunosuppression. In: PLoS ONE . Volume 7, number 12, 2012, p. E49897, doi: 10.1371 / journal.pone.0049897
- ↑ a b c d e f g h i j k l m D. E. Rusyniak: Neurologic manifestations of chronic methamphetamine abuse. In: The Psychiatric clinics of North America. Volume 36, Number 2, June 2013, pp. 261-275, doi: 10.1016 / j.psc.2013.02.005
- ↑ AL Cohen, C. Shuler, S. McAllister, GE Fosheim, MG Brown, D. Abercrombie, K. Anderson, LK McDougal, C. Drenzek, K. Arnold, D. Jernigan, R. Gorwitz: Methamphetamine use and methicillin- resistant Staphylococcus aureus skin infections. In: Emerging Infectious Diseases . Volume 13, number 11, November 2007, pp. 1707-1713, doi: 10.3201 / eid1311.070148
- ↑ RE Pecha, T. Prindiville, BS Pecha, R. Camp, M. Carroll, W. Trudeau: Association of cocaine and methamphetamine use with giant gastroduodenal ulcers. In: The American Journal of Gastroenterology. Volume 91, Number 12, December 1996, pp. 2523-2527, PMID 8946979 .
- ↑ LE Halpin, SA Collins, BK Yamamoto: Neurotoxicity of methamphetamine and 3,4-methylenedioxymethamphetamine. In: Life Sciences . Volume 97, number 1, February 2014, pp. 37-44, doi: 10.1016 / j.lfs.2013.07.014
- ↑ DE Rusyniak: Neurologic manifestations of chronic methamphetamine abuse. In: Neurologic clinics. Volume 29, number 3, August 2011, pp. 641-655, doi: 10.1016 / j.ncl.2011.05.004
- ^ BD Marshall, D. Werb: Health outcomes associated with methamphetamine use among young people: a systematic review. In: Addiction. Volume 105, Number 6, June 2010, pp. 991-1002, doi: 10.1111 / j.1360-0443.2010.02932.x
- ^ JF Marshall, SJ O'Dell: Methamphetamine influences on brain and behavior: unsafe at any speed? In: Trends in neurosciences. Volume 35, Number 9, September 2012, pp. 536-545, doi: 10.1016 / j.tins.2012.05.006
- ↑ KM Grant, TD LeVan, SM Wells, M. Li, SF Stoltenberg, HE Gendelman, G. Carlo, RA Bevins: Methamphetamine-associated psychosis. In: Journal of Neuroimmune Pharmacology : the official journal of the Society on NeuroImmune Pharmacology. Volume 7, Number 1, March 2012, pp. 113-139, doi: 10.1007 / s11481-011-9288-1
- ↑ "Crystal". Drugstore project Germany
- ^ Thomas Nordegren: The AZ Encyclopedia of Alcohol and Drug Abuse. Universal Publishers, 2002, ISBN 978-1-58112-404-0 , p. 357 ( limited preview in Google book search).
- ↑ Burkhard Madea: Practice forensic medicine. Springer-Verlag, 2013, ISBN 978-3-662-09424-2 , p. 337 ( limited preview in the Google book search).
- ↑ Aleksei F. Bobkov, Ludmila M. Selimova, Tatyana A. Khanina, Sergey Y, Zverev, Vadim V. Pokrovsky, Jonathan N. Weber, Eugene N. Bobkov, Andrey V. Rylkov: Human immunodeficiency virus type 1 in illicit-drug solutions used intravenously retains infectivity. In: Journal of clinical microbiology. 2005, Volume 43 (4), pp. 1937-1939. doi: 10.1128 / JCM.43.4.1937-1939.2005
- ↑ Sisa: Cocaine of the Poor. In: Vice . May 16, 2013, accessed July 4, 2016 .
- ↑ Fragkiska Megaloudi: Crisis Changes Habits of Drug Addicts: Death Toll Rising in Greece. January 7, 2013, accessed July 4, 2016 .
- ↑ Alex Rühle: Sisa addicts in Greece - fight against the AIDS explosion. In: sueddeutsche.de. June 23, 2013, accessed July 4, 2016 .
- ↑ Sisa: The "cocaine of the poor" . Deutschlandfunk , June 26, 2019; accessed on August 8, 2019
- ^ Methamphetamine: a European Union perspective in the global context. (PDF) EMCDDA , Europol , Lisbon, July 2009 (PDF; 1.6 MB).
- ↑ a b Falk Harnisch, Tunga Salthammer: The chemistry at Breaking Bad. A chemist as a series protagonist. In: Chemistry in Our Time . Volume 47, Issue 4, 2013, pp. 214–221, doi: 10.1002 / ciuz.201300612
- ↑ Bmg: making crystal meth more difficult. (No longer available online.) In: drogenbeauftragte.de. October 13, 2016, archived from the original on October 11, 2016 ; accessed on November 2, 2016 .
- ↑ Irving W. Wainer, Dennis E. Drayer: Drug Stereochemistry. Marcel Dekker, New York and Basel 1988, pp. 209–368, ISBN 0-8247-7837-5 .
- ↑ K. Uekusa, M. Hayashida, N. Saito, K. Mashiko, K. Hara, B. Waters, Y. Ohno: Methamphetamine and amphetamine concentrations in survivors of body-packer syndrome in Japan. In: Forensic Science International. April 10, 2013, Vol. 227 (1-3), pp. 45-47, PMID 23116635 .
- ↑ MS Castaneto, AJ Barnes, KB Scheidweiler, M. Schaffer, KK Rogers, D. Stewart, MA Huestis: Identifying methamphetamine exposure in children. In: Therapeutic Drug Monitoring . Volume 35 (6), 2013. pp. 823-830, PMID 24263642 .
- ↑ O. Beck: Exhaled breath for drugs of abuse testing - evaluation in criminal justice settings. In: Science and Justice. Volume 54 (1), 2014. pp. 57-60, PMID 24438778 .
- ↑ PM O'Byrne, PV Kavanagh, SM McNamara, SM Stokes: Screening of stimulants including designer drugs in urine using a liquid chromatography tandem mass spectrometry system. In: Journal of Analytical Toxicology . Volume 37 (2), 2013. pp. 64-73, PMID 23316030 .
- ↑ W. Wongniramaikul, A. Choodum, L. Dennany, N. Nic Daeid: A comprehensive chromatographic comparison of amphetamine and methylamphetamine extracted from river water using molecular imprinted polymers and without the need for sample derivatization. In: Journal of Separation Science . Volume 35 (23), 2012. pp. 3332-3339, PMID 23184370 .
- ↑ EU drugs agency (EMCDDA) and Sewage analysis CORe group - Europe (SCORE): Wastewater analysis and drugs - a European multi-city study. emcdda.europa.eu . Lisbon, March 7th 2018.
- ↑ Twenty-first Narcotics Law Amendment Ordinance - 21st BtMÄndV. February 18, 2008, accessed January 27, 2017.
- ↑ 10. BtMÄndV Art. 1 No. 1 letter b; Art. 1 No. 3; Art. 3 (PDF).
- ↑ Press release No. 228/08 of the BGH from December 9, 2008 . BGH judgment Az .: 2 StR 86/08 of December 3, 2008, guiding principle and from p. 11.
- ↑ § 29a para. 1 no. 2, § 30 para. 1 no. 4 and § 30a BtMG
- ^ Judgment of the Federal Court of Justice of November 17, 2011, Az .: 3 StR 315/10 , judgment discussion on Rechtslupe.de
- ^ RIS - Federal Law Consolidated - Search .
- ↑ SR 812.121.11 Ordinance of the EDI of 30 May 2011 on the registers of narcotics, psychotropic substances, precursor substances and auxiliary chemicals (Narcotics Directory Ordinance, BetmVV-EDI) .
- ↑ SR 812.121 Federal Act of October 3, 1951 on Narcotics and Psychotropic Substances (Narcotics Act, BetmG) .
- ↑ 'Breaking Bad' shows bygone meth era, Utah law enforcement says. In: The Salt Lake Tribune , August 13, 2013, accessed September 29, 2013.


