boiling point
The boiling point (abbreviation: . Bp ), evaporation point or boiling point (abbreviation: . Kp ) of a pure substance is a pair of values in the phase diagram and is composed of two variables : the saturation temperature (especially boiling temperature ) and the saturation vapor pressure (especially boiling pressure ) at the Phase boundary line between gas and liquid . Thus it is composed of the two state variables of pressure and temperature at the transition of a substance from a liquid to gaseous state of aggregation together. In an open liquid, the boiling point is therefore the point on the temperature scale at which the vapor pressure equals atmospheric pressure.
The boiling point represents the conditions that exist during the phase transition of a substance from the liquid to the gaseous phase , which is known as boiling . In addition, it is identical to the condensation point for the reverse process of condensation , but only for pure substances . When a mixture of substances evaporates, the boiling behavior changes, and a boiling range is observed instead of a single boiling point. A phase transition from the liquid to the gaseous phase below the boiling point is known as evaporation .
The boiling temperatures are given in tables at normal pressure , i.e. at 1013.25 hPa . This boiling point is used as a normal boiling point , the boiling temperature as specified Normalsiedetemperatur ( T Sied hereinafter). One method for estimating this is the Pailhes method , while the Guldberg rule establishes a connection with the critical temperature . The term boiling point is often used as a short form for the normal boiling temperature and is therefore usually a synonym in common usage , which, however, would reduce the boiling point to a single pair of values and is therefore formally incorrect.
A pressure cooker , for example, makes use of the fact that the boiling temperature and the boiling pressure are interdependent. By increasing the pressure by usually one bar (1000 hPa), the boiling temperature of the water can be increased from 100 ° C to around 120 ° C. Both temperatures represent boiling temperatures, but only the value of 100 ° C is also the boiling temperature under normal pressure and thus the normal boiling temperature. Mixing the two terms is therefore unspecific, by no means self-evident and should be avoided.
Boiling process
Below and above the boiling point, heating the liquid or gas only leads to an increase in temperature . The supplied energy is used in kinetic energy converted the particles. During the phase transition of the liquid to the gas, however, the temperature remains constant, provided that the pressure also remains constant. All thermal energy supplied is invested in the change of state .
Once the boiling point has been reached, the chemical-physical interactions between the particles are dissolved when further energy is supplied - the particles pass into the gas phase. The temperature of the liquid stagnates because the thermal energy supplied is fully used to break the intermolecular bonds . The energy that is required for one mole of the substance is also referred to as the enthalpy of evaporation and its counterpart , which is not related to the amount of substance, as the heat of evaporation . Only when all the particles are in the gas phase does the temperature of the system rise again.
Water, hydrogen peroxide or alkalis ( e.g. caustic soda ) without dust particles or gas bubbles can also be heated above the boiling point in clean vessels without boiling. The smallest disturbances, such as vibrations that result in thorough mixing, can lead to an explosive separation of the liquid from the vapor phase, which is known as boiling delay . To avoid this, liquids that tend to delay boiling, so-called boiling stones made of clay or pumice stone, are added in chemical work, which are not attacked by the chemical, but facilitate the formation of small bubbles due to their porous structure so that there is no delay in boiling.
See also : evaporation , gasification , evaporation , transpiration , Pictet-Trouton rule
Boiling point curve
All temperature-pressure value pairs at the gas-liquid phase boundary line in a phase diagram result in the boiling point curve , with a thermodynamic equilibrium prevailing on it . The boiling point curve is often also referred to as the boiling curve , boiling line , boiling pressure curve or boiling point curve . This curve is limited by two points:
- Triple point P t : If the pressure-temperature value pair is lower than the triple temperature or the triple pressure, then only a transition between solid and gaseous state, i.e. sublimation or resublimation, is possible.
- Critical point P c : If the pressure-temperature value pair is higher than the critical temperature or the critical pressure, there is no longer any difference between the density of the liquid and that of the gaseous state, which is why they are no longer separated by a phase boundary line Substance in this state is therefore called supercritical fluid .
The equilibrium of the boiling point curve is a dynamic equilibrium . Particles from a liquid constantly pass into the gas phase - they evaporate. On the other hand, these particles also re-enter the liquid phase - they condense. The numerical ratio of the particles emerging from the liquid phase and the particles entering it again depends on both the temperature and the pressure : the higher the temperature, the more particles evaporate due to their higher speed (see Maxwell-Boltzmann distribution ) . The more particles that evaporate, the higher the vapor pressure and the more particles that condense again. An equilibrium is established when the same number of particles enter the gas phase as they return to the liquid phase. Since the gas phase is saturated in this state , one speaks of the saturation vapor pressure . The thermodynamic law from which the boiling point curve is quantitatively derived is known as the Clausius-Clapeyron equation . For water, this relationship between saturation vapor pressure and saturation temperature can also be determined using the approximation equations of the Magnus formula .
Change of equilibrium using the example of water
Exemplary starting point: water is in equilibrium with its gas phase at a boiling point of 80 ° C and a pressure of 474 hPa:
The reactions of the system to the changes in individual state variables result in a shift in the equilibrium position: the phase transition that reverses the disturbance takes place more intensely (see principle of the smallest compulsion ).
- If the system is cooled to 70 ° C, the vapor pressure of the gas phase is too high and water vapor condenses until the vapor pressure has the new equilibrium value of 312 hPa or there is no gaseous water left.
- If the system is heated to 90 ° C, the vapor pressure of the gas phase is too low and water evaporates until the vapor pressure reaches the new equilibrium value of 702 hPa or there is no more liquid water left.
- If the pressure is increased from 474 to 700 hPa while the temperature remains the same, the vapor pressure of the gas phase is too high and gaseous water condenses until the vapor pressure has the old equilibrium value of 474 hPa or there is no more water vapor left.
- If the pressure is reduced from 474 to 250 hPa while the temperature remains the same, the vapor pressure of the gas phase is too low and water evaporates until the vapor pressure has the old equilibrium value of 474 hPa or there is no more liquid water left.
Substance dependence of the boiling point
- The boiling point depends on the molar mass or molecular mass of the substance. The following applies: the greater the molar mass, the higher the boiling point. If one compares, for example, the series HCl (36 g / mol) - HBr (81 g / mol) - HI (128 g / mol) on the dark blue line, this relationship can be clearly seen. Explanation: The greater the mass of a particle, the more kinetic energy it needs to be able to pass into the gas phase.
- The boiling point is also dependent on the strength of the binding forces between the smallest particles in the liquid phase: the stronger the binding forces, the higher the boiling point, as they have to be overcome first. This becomes clear when comparing HF and HCl, for example: In liquid hydrogen fluoride, the molecules form hydrogen bonds , while in liquid hydrogen chloride the weaker dipole-dipole interactions predominate. The same applies to the comparatively very high boiling point of water, which becomes clear when you compare it with carbon dioxide and take into account the influence of the molar masses.
- The observation that substances have a higher boiling point than similar substances with a higher molar mass is known as a boiling point anomaly.
- London dispersion interactions are even weaker than dipole-dipole interactions . For this reason, all hydrogen compounds of the elements of main group IV have the lowest boiling points when compared .
- The strength of the intermolecular binding forces also depends on the geometry of the molecules. See the boiling points of the homologous series of hydrocarbons or alcohols .
Examples of normal boiling points of pure substances
Chemical elements
- Helium has the lowest normal boiling temperature of all elements with -269 ° C , although it has a larger molar mass than hydrogen with a normal boiling temperature of -253 ° C. This is due to the fact that the hydrogen molecule is somewhat easier to polarize than helium and therefore also forms somewhat stronger van der Waals interactions.
- The highest normal boiling temperature is given inconsistently in the literature. Tungsten and rhenium are both above 5000 ° C.
- A group comparison of noble gases , non-metals , semimetals and metals shows that metals have a significantly higher boiling point than non-metals, since the metal bond (in addition to the ionic and atomic bonds) is the strongest bond. Exceptions:
- Mercury has a normal boiling temperature of 357 ° C, which is unusually low for metals
- Carbon has an extremely high boiling point of 4827 ° C for non-metals.
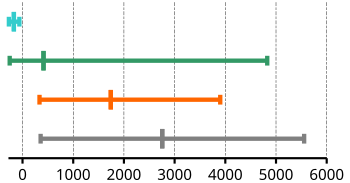
| Boiling points | ||||
|---|---|---|---|---|
| minimum | maximum | average | Graphic illustration | |
| Noble gases | −269 | −62 | −170.5 | |
| Non-metals | −253 | 4827 | 414.1 | |
| Semi-metals | 335 | 3900 | 1741.5 | |
| Metals | 357 | > 5000 | 2755.9 | |
links
Carbon monoxide has one of the lowest normal boiling temperatures at -191.6 ° C, the highest are metal carbides such as titanium (IV) carbide ( TiC , 4820 ° C) and tungsten (IV) carbide ( WC , 6000 ° C).
Sulfur trioxide (SO 3 ) has a special feature : the melting point of one of its modifications is 62.3 ° C above the normal boiling temperature of 44.8 ° C of liquid sulfur trioxide.
If the critical pressure is below normal pressure, no normal boiling temperature can be specified. In order to still bring the liquid to the boil, this must be done under lower pressure. In this case, when specifying the boiling temperature, the boiling pressure must also be specified, which is another reason for strictly separating the terms normal boiling temperature and boiling point.
If the pressure of the triple point is above normal pressure, the normal sublimation temperature or a boiling temperature at a higher boiling pressure is given instead of the normal boiling temperature. Example: Sulfur hexafluoride SF 6 sublimes under normal pressure at -63 ° C.
Many, especially organic and all macromolecular compounds decompose when heated before the boiling point is reached, as their enthalpy of vaporization is greater than the individual binding energies in the molecule. You cannot specify a boiling point here, only the decomposition temperature . However, some can be brought to the boil under reduced pressure and thus at a lower temperature.
Homogeneous multi-component systems
The boiling points of homogeneous mixtures such as alloys , gas mixtures or aqueous solutions have different boiling points and a different boiling behavior compared to the pure substances.
Boiling point increase
If a substance is dissolved in a solvent , the boiling point of the mixture increases compared to the pure solvent; in relation to the saturation vapor pressure one speaks of the solution effect . According to Raoult's law of François Marie Raoult (1830–1901), this increase ΔT Sdp is proportional to the amount of substance in the dissolved substance:
| solvent | ebullioscopic constant in K kg / mol |
|---|---|
| water | 0.513 |
| Methanol | 0.86 |
| Ethanol | 1.23 |
| phenol | 3.54 |
| acetic acid | 3.22 |
| benzene | 2.64 |
| Carbon disulfide | 2.42 |
| Carbon tetrachloride | 5.26 |
| Cyclohexane | 2.92 |
The individual symbols stand for the following quantities :
- ΔT Sdp - Boiling Point Increase
- K e - ebullioscopic constant
- b - molality of the solute
- K - molar boiling point increase
- n - amount of substance
The proportionality factor is, as stated, the ebullioscopic constant (also boiling point constant K S ), ie the change in the boiling point of one kilogram of the solution compared with the pure solvent, wherein the molar amount of the solute a mole amounts or the molar elevation of boiling point , which is less common and makes no statement about the mass.
For example, the boiling point of one kilogram of water increases by 0.51 K to 100.51 ° C if exactly one mole of any other substance is dissolved in it, provided that the substance dissolves in water and is not volatile. If you dissolve two moles in one kilogram of water, the water only boils at 100 ° C + 2 × 0.51 ° C = 101.02 ° C.
It should be noted that salts dissociate in aqueous solution . For example, sodium chloride breaks down into the ions Na + and Cl - . The increase in boiling point is therefore (in dilute solutions) twice as high as initially expected.
A practical example: pasta water has a typical salt content of 10 g / kg. With a molar mass of 58.4 g / mol, this, together with the doubling mentioned above, corresponds to 0.34 mol / kg of ions. The salt content results in a boiling point increase of only about 0.17 K.
Raoult's law only applies to "ideal" solutions, those are solutions in which a substance is only physically dissolved. In the case of “non-ideal” solutions, energetic phenomena (heating or cooling) occur during mixing, which are caused by the formation of hydrogen bonds or by protolysis . This results in deviations from Raoult's law. Only in very strong dilutions does the formula also apply to "non-ideal" approximate solutions, which is why one speaks of an infinitely diluted solution in the case of the ideal solution . The increase in boiling point is also a colligative property and therefore depends on the number of particles in the dissolved substance, but not on its type. By changing the above formula, the increase in boiling point can also be used to determine molar mass , which is known as ebullioscopy .
The melting point is also dependent on the concentration of the dissolved substances , which is why one speaks of a lowering of the melting point . The cause of these effects is also a lowering of the chemical potential . If you combine an increase in the boiling point and a decrease in the melting point, an overall expansion of the thermodynamic state of the liquid at the expense of the other states of aggregation is evident.
Boiling ranges
Zeotropic mixtures
If a zeotropic mixture of two liquids is heated, it begins to boil at a temperature above the boiling point of the low boiler , i.e. the component with the lower boiling point. When boiling, both components go into the gas phase at the same time. The low boiler has a higher concentration in the emerging vapor than its concentration in the liquid mixture. Therefore, the high boiler accumulates in the liquid and the boiling temperature rises continuously to the boiling point of the high boiler. In this case, one speaks of a boiling range (also boiling interval, boiling limit) of the mixture and no longer of a boiling point.
The dependence of the boiling temperature on the ratio of the liquid components and the corresponding ratio in the evaporating gas are shown in the boiling diagram (right). The free area in the middle, in which neither gas nor liquid can exist, is called the boiling lens because of its shape . The boiling range of a solution with 50% molar content is marked on the right edge .
This behavior is used technically to increase the concentrations of individual components in mixtures through distillation or rectification .
Azeotropic mixtures

Boiling diagram of azeotropic mixtures Bp. 1: Boiling point of the pure substance component 1, Bp. 2: Boiling point of the pure substance component 2 , x: Mole fraction of component 2 in the azeotropic mixture
In the case of azeotropic substance mixtures, the boiling point of the substance mixture has an extreme value at a certain molar ratio . This value is outside the temperature range that is spanned by the boiling temperatures of the two pure substances. With this particular mixing ratio, there is a boiling point and not a boiling range.
Examples:
- Water (bp 100 ° C) and HCl (bp - 85 ° C) - azeotropic mixture with 20.2% HCl: bp 108.6 ° C
- Water (bp 100 ° C) and ethanol (bp 78.3 ° C) - azeotropic mixture with 96% ethanol: bp 78.2 ° C
Importance for living beings
Under the physical conditions on earth, the boiling behavior of water means that water exists in large quantities as a liquid. This is one of the basic requirements for the development of living beings .
With a lower air pressure or higher water temperatures, this would be different and would result in bodies of water evaporating within a very short time and thus an important condition for life in general, namely liquid water, would be encountered much less often. At a higher air pressure or a lower temperature, however, less and less water would be able to evaporate, and thus the prerequisite for precipitation, namely gaseous water in the atmosphere, would become increasingly rare, which would, for example, result in a restriction of fresh water resources.
Applications
- Analytical chemistry : the boiling point is a specific property of a substance . In this way, pure substances can be characterized based on their boiling point.
- Distillation or fractional distillation , a method for separating a mixture of substances based on the different boiling points of the individual components. The low-boiling material is separated from the higher-boiling material by evaporation.
- The ebullioscopy (lat. Bulla = Siedeblase, gr. Skopein look =) is a method for the determination of the molar masses by boiling point elevation. Since increases in the boiling point are smaller than decreases in the freezing point , cryoscopy is usually preferred. Both methods use a special thermometer that was developed in 1888 by Ernst Otto Beckmann (1853–1923): the Beckmann thermometer . It has a scale that is only about 6 °, but can also be read to within 0.01 degrees. The zero point of the scale can be set to the required temperature.
- Pressure cooker : If water is heated in an airtight pot, the temperature of the liquid water can rise above 100 ° C because the boiling pressure and thus the boiling point increase. This results in faster cooking.
- Altitude measurement : As the air pressure decreases with increasing altitude, the boiling point also decreases. As a rule of thumb, the boiling point is lowered by about one degree per 300 m. By determining the boiling temperature of pure water, it is possible to estimate the respective altitude above mean sea level .
Determination of the boiling points of organic substances
The boiling point is a material property. Knowing the boiling point enables conclusions to be drawn about the substance in question. Reference works (e.g. CRC Handbook of Chemistry and Physics or Pocket Book for Chemists and Physicists) contain tables on the boiling points of substances and mixtures of substances. The suspected connections can often be estimated from the table values.
The boiling point can also be used as a criterion for the purity of a known substance. A Vigreux column is suitable for the separation of substances by distillation.
In the case of mixtures of substances, it can also happen that several substances distill over at the same boiling point, the mixture of substances then forming an azeotrope .
Determination of the boiling point with simple means
For simple determination of the boiling temperature you need a test tube (or a flask) with a side tube with a pierced rubber stopper and thermometer, a piece of rubber hose, a glass tube, a paraffin bath, a heat source and a beaker with a cooling tank. A corresponding distillation device is set up according to the illustration of the apparatus for determining the boiling point .
The test tube with tube should be filled to one third with the substance to be examined. To prevent delays in boiling, a few small boiling stones are added. The lower tip of the thermometer should be a few centimeters above the surface of the liquid. Instead of a mercury thermometer, a digital temperature sensor (accuracy: 0.1 ° C) can be inserted through the rubber stopper for more precise temperature determination.
For a very exact determination of the boiling point, two possible sources of error must be considered:
- If a mercury thermometer is used to determine the boiling point, the thermometer must protrude very far into the test tube. The significantly cooler ambient air cools the mercury that is not in the interior of the vapor zone. The resulting temperature error is approximately described by the formula Δ T = 0.000154 · n · ( T - t ). ( n = length (cm) of the protruding section, T = observed boiling temperature, t = temperature of the ambient air).
- If the air pressure deviates from 760 mm of mercury, another correction factor for the boiling point must be taken into account. Roughly, a pressure deviation of 0.36% (2.4 mm mercury column) from normal pressure leads to a boiling point deviation of at least 0.1 ° C.
Individual evidence
- ↑ ChemgaPedia: Glossary: Boiling point .
- ↑ Pure Appl. Chem. , Vol. 54, No. 6, pp. 1239-1250, 1982. Full text (PDF file; 227 kB).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Ebullioscopic Constants for Calculation of Boiling Point Elevation, pp. 15-27.
- ^ S. Scholl, Henning Föste: Process engineering laboratory . Rectification. ( PDF; 2.1 MB [accessed November 9, 2013]).
- ↑ Ber. d. Deut. Chem. Ges. 22 , 3072.
- ↑ Ber. d. Deut. Chem. Ges. 20 , 709.