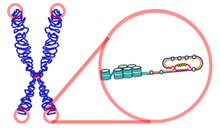
Telomere
| Parent |
| chromosome |
| Subordinate |
| Protein complexes |
| Gene Ontology |
|---|
| QuickGO |
The telomeres ( Gr. Τέλος télos "end" and μέρος méros "part") are the ends of linear chromosomes consisting of repetitive DNA and associated proteins . The repeated sequence ( repeat sequence ) and caused by this repeat length are similar in different organisms, often the same. In vertebrates, "hexanucleotides", i.e. six nucleotides , repeat themselves several thousand times with the sequence 5'-TTAGGG-3 '. Telomeres are accordingly several kilobase pairs (kbp) long. As essential structural elements, these end pieces stabilize your chromosome. The folded secondary structure of the telomere DNA is also important for the stabilization effect.
Structure of the telomeres
The chromosomes of many eukaryotes have a typical motif rich in thymine and guanine , which is assumed to form a quadruple helix . First the 3 'overlapping strand pairs with itself and forms abnormal GG double bonds. This double strand mates with itself again and thus forms the quadruple helix, in which the guanines form so-called Hoogsteen bonds . The sequence of the telomere DNA is recognized and bound by the DNA-binding protein complex Shelterin . The protein complex enables the cell to distinguish the natural chromosome end from broken DNA strands. Cytologically, telomeres belong to heterochromatin because of their tight packaging .
The repeating unit 5'– TTAGGG – 3 'known from vertebrates was also found in the original metazoa : in sponges , cnidarians , comb jellyfish and plate animals . That is why this conserved sequence unit represents the original telomeric motif. The same telomeric motif is also shown on human chromosomes. The telomeres in insects have also been well studied.
Telomeres in replication
Organisms with telomerase
With each cell division , the telomeres become shorter, as the (normal) DNA polymerase can no longer attach to the subsequent strand . The telomerase compensates for the shortening of the DNA ends off. This enzyme is an RNA - protein complex that functions as a specialized reverse transcriptase . To do this, she adds G-rich repeat units to the 3'-OH end, the RNA of which is located in the telomerase itself. “It works like building a bridge, which is driven by a self-supporting structure.” Then the DNA strand folds over and forms abnormal GG base pairings with itself. From this point the RNA primase and the DNA polymerase can fill in the next strand (also called discontinuous reverse strand ).
Telomerase is active in principle in unicellular eukaryotes ( protozoa ). In higher, multicellular organisms , however, the enzyme is only used in very specific cells after the embryonic stage :
- in the cells of the germ line ,
- in cells that have to divide very often, such as stem cells (e.g. in the bone marrow ) and immune cells ,
- in up to 94% of all proliferating cancer cells .
The enzyme activity of telomerase can be determined using the TRAP method.
If the telomere length falls below a critical minimum of around 4 kbp, the cell can no longer divide. Then programmed cell death ( apoptosis ) or a permanent growth stop ( senescence ) often occurs . The cell's lifespan, which is limited by this, is understood as a mechanism for tumor suppression . If the cells continue to divide despite the shortened telomeres, as in some cancer cells , the chromosomes lose their stability . However, it has been shown in knock-out mice that they remain viable for several generations without telomerase. It is believed that telomeres can also be repaired through recombination events; this has not yet been clarified in mammals.
Organisms without telomerase
The five long polytene chromosomes of Drosophila hydei (1n = 6) showed large end structures under the electron microscope. These compact regions were considered to be the “morphological manifestation of the postulated telomeres”. It came as a surprise to learn that the genetics model fly does not have a telomerase at all. The ends of the chromosomes of Drosophila melanogaster consist of repeats of specialized retrotransposons . The length of such telomeres is guaranteed by transposition . In the telomeres of the polytene chromosomes of D. melanogaster , three retrotransposon regions can be distinguished, namely (1) cap, (2) HeT-A / TAHRE / TART and (3) repetitive TAS. Each of the three regions binds its own proteins; the three domains do not overlap. The telomeres of this fruit fly have also been examined in mitotic interphase nuclei , namely in the syncytial blastoderm , in which the divisions take place synchronously. Of particular interest were the telomeres of the Y chromosome. Because except in the male germ line , Drosophila-Y is entirely heterochromatic.
In addition to the fruit flies, mosquitoes also have an alternative mechanism for elongating their telomeres, namely unequal recombination . It was therefore suggested that an ancestor of the Diptera lost telomerase. Telomeres with repeating units that differ from those of the telomerase organisms have also been found in several species of beetles and Schnabelkerfen .
Mary-Lou Pardue explained that it makes no difference whether the telomeres are lengthened by telomerase or by retrotransposition. Reverse transcriptase is involved in both enzyme methods. In the case of the retroposon telomeres, the entire RNA is copied as an intermediate product of the transposition. Telomerase works more elegantly because it only copies the telomeric repeat unit from its RNA template.
Importance of telomeres
When Barbara McClintock and Hermann Joseph Muller examined broken chromosomes, they recognized for the first time how important the ends of linear chromosomes are for their stability. The two American Nobel Prize winners are the originators of the term and word telomer (Greek for end part). In addition to the two telomeres, each chromosome needs a centromere and at least one starting point for DNA replication in order to survive in a cell nucleus.
Telomeres preserve linear chromosomes on both sides during cell cycles and are therefore important for all biological processes. They have been associated with the aging of cells and with their immortalization and also with the development of cancer .
Influences
The influence of chronic stress on the accelerated shortening of the telomeres is mediated by the balance of news substances ( neurotransmitters ) dopamine and serotonin . Moderate lifestyle changes can slow the shortening of telomeres.
Recent research
An examination of the genome of the spaceman Scott Joseph Kelly , who was in space for almost a year from 2015 to 2016 , found that Scott's telomere ends had become significantly longer in space, but returned to their original length immediately after his return to Earth assumed. The purpose of this phenomenon is so far unknown.
Telomeropathy
Mutations in the genes for proteins that are responsible for the protection, "maintenance" and repair of the telomeres result in significantly shortened telomeres. This applies above all to the shelterine complex and the telomerase complex. This results in a significantly reduced pool of stem cells with a lower regenerative "quality", which trigger a group of chronic diseases known as telomere diseases or telomeropathies :
- Aplastic anemia with bone marrow failure and pancytopenia
- Forms of myelodysplastic syndrome
- Idiopathic pulmonary fibrosis
- idiopathic cirrhosis of the liver
- Dyskeratosis congenita with numerous subtypes and different mutations of genes that all encode proteins that have a function on the telomeres. The function and importance of the telomeres and their proteins was only recognized through the development of this syndrome.
Telomeropathies are genetically very heterogeneous with high variability in penetrance .
Androgens were successfully used to treat bone marrow failure as early as the 1960s . Experiments in vitro have on human lymphocytes and human CD 34-positive hematopoietic stem cells show that androgens gene expression for reverse telomerase - transferase (TERT) and the enzymatic can increase telomerase activity. In mice with telomere insufficiency, this can even lead to a haematological improvement and an increase in telomere length.
A first prospective phase 1/2 study with the administration of the synthetic androgen danazol (800 mg daily for 24 months) had to be stopped prematurely because of the unexpectedly strong effect, because the primary endpoint had been reached in an interim analysis in all twelve patients that could be evaluated by then , the telomere damage did not increase any further. In eleven patients, the telomeres were even longer after 24 months of therapy than at the beginning (92%) with a mean lengthening of 386 base pairs, mainly in the first year of treatment. In 19 of the 24 patients (79%) who had been treated for at least three months before the study was stopped, a haematological improvement was found. While 13 patients required regular blood transfusions before the start of the study , this was only one patient when the study stopped.
This study gives rise to a larger randomized controlled study , but from this small study with only a few participants no therapeutic recommendation for practice can be derived, since above all risks, long-term and adverse effects could not be recorded.
Fiction
In 1999 the American writer John Darnton treated the subject of telomeres in his novel Zwillingspark (The Experiment).
literature
- ES Epel, EH Blackburn et al. a .: Accelerated telomere shortening in response to life stress. In: Proc Natl Acad Sci USA . Volume 101, No. 50, December 14, 2004, pp. 17323-17324.
- M. Mills, L. Lacroix, et al. a .: Unusual DNA conformations: Implications for telomeres. In: Current Medicinal Chemistry - Anti-Cancer Agents. Volume 2, No. 5, September 2002, pp. 627-644.
- Guenther Witzany: The viral origins of telomeres, telomerases and their important role in eukaryogenesis and genome maintenance. In: Biosemiotics. Volume 1, 2008, pp. 191-206.
Web links
- [2] Nobel Prize 2009 to Elizabeth H Blackburn, Carol W Greider and Jack W Szostak.
- medicineprize2009.pdf Summary by Rune Toftgård.
Individual evidence
- ↑ Elizabeth H Blackburn , Joseph G. Gall : A tandemly repeated sequence at the terms of the extrachromosomal ribosomal RNA genes in Tetrahymena. In: J Mol Biol 120 (1), 1978: 33-53.
- ^ Jack Szostak , Elizabeth H Blackburn: Cloning yeast telomeres on linear plasmid vectors. In: Cell 29 (1), 1982: 245-255.
- ↑ S Perrod, SM Gasser: Long-range silencing and positional effects at telomeres and centromeres: parallels and differences. In: Cell Mol Life Sci 60, 2003: 2303-2318.
- ^ Walther Traut, Monika Szczepanowski, Magda Vítková, Christian Opitz, František Marec, Jan Zrzavý: The telomere repeat motif of basal Metazoa. In: Chromosome Research 15, 2007: 371-382 doi: 10.1007 / s10577-007-1132-3 .
- ↑ RK Moyzis, JM Buckingham, LS Cram, LL Deaven, MD Jones, J Meyne, RL Ratliff, JR Wu: A highly conserved repetitive DNA sequence, (TTAGGG) n, present at the telomeres of human chromosomes. In: Proc Natl Acad Sci USA 85 (18), 1988: 6622-6626.
- ↑ Magda Vítková, Jiří Král, Walther Traut, Jan Zrzavý, František Marec: The evolutionary origin of insect telomeric repeats, (TTAGG) n. In: Chromosome Research 13, 2005: 145-156.
- ↑ Gregg B Morin: The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. In: Cell 59, 1989: 521-529.
- ↑ Charles Schumpert, Jacob Nelson, Eunsuk Kim, Jeffry L Dudycha, Rekha Patel C: telomerase activity and telomere length in Daphnia. In: PLoS One 10 (5), 2015: e0127196. PMC 4427308 (free full text)
- ↑ Stephan Jentsch, Heinz Tobler, Fritz Müller: New telomere formation during the process of chromatin diminution in Ascaris suum. In: Int J Dev Biol 46 (1), 2002: 143-148. [1] Open article.
- ^ Alan M Zahler, David M Prescott: DNA primase and the replication of the telomeres in Oxytricha nova. In: Nucleic Acids Research 17 (15), 1989: 6299-6317.
- ↑ Carol W Greider , Elizabeth H Blackburn: A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. In: Nature 337 (6205), 1989: 331-337.
- ↑ Helmut Zacharias, Inge Kronberg: Telomeres: All's well that ends well. In: Biology in our time 6, 2009: 366–367 doi: 10.1002 / biuz.200990086 .
- ↑ Hans D Berendes, Günther F Meyer: A specific chromosome element, the telomere of Drosophila polytene chromosomes. In: Chromosoma 25 (2), 1968: 184-197.
- ↑ Harald Biessmann, James M Mason, K Ferry, M d'Hulst, K Valgeirsdottir, KL Traverse, Mary-Lou Pardue: Addition of telomere-associated HeT DNA sequences “heals” broken chromosome ends in Drosophila. In: Cell 61 (4), 1990: 663-673.
- ↑ Harald Biessmann, Larry E Champion, Mitch O'Hair, Karen Ikenaga, Babak Kasravi, James M Mason: Frequent transpositions of Drosophila melanogaster HeT-A transposable elements to receding chromosome ends. In: EMBO Journal 11 (12), 1992: 4459-4469. PMC 557021 (free full text)
- ↑ Fang-Miin Sheen, Robert W Levis: Transposition of the LINE-like retrotransposon TART to Drosophila chromosome termini. In: Proc Natl Acad Sci USA 91, 1994: 12510-12514. PDF
- ↑ Mary-Lou Pardue: Drosophila telomeres: Another way to end it all. In: Elizabeth H Blackburn, Carol W Greider (eds.): Telomeres. CSH Laboratory Press, Cold Spring Harbor, New York 1995: 339-370.
- ↑ Evgenia N Andreyeva, Elena S Belyaeva, Valerii F Semeshin, Galina V Pokholkova, Igor F Zhimulev: Three distinct chromatin domains in telomere ends of polytene chromosomes in Drosophila melanogaster Tel mutants. In: J Cell Science 118, 2005: 5465-5477. doi: 10.1242 / jcs.02654 PDF
- ↑ Natalia Wesolowska, Flavia L Amariei, Yikang S Rong: Clustering and protein dynamics of Drosophila melanogaster telomeres. In: Genetics 195 (2), 2013: 381-391. PMC 3781967 (free full text).
- ↑ Sidney H Wang, Ruth Nan, Maria C Accardo, Monica Sentmanat, Patrizio Dimitri, Sarah CR Elgin: A distinct type of heterochromatin at the telomeric region of the Drosophila melanogaster Y chromosome. In: PLOS ONE 9 (1), 2014: e86451. doi: 10.1371 / journal.pone.0086451
- ↑ Lena Nielsen, Jan-Erik Edström: Complex telomere-associated repeat units in members of the genus Chironomus evolve from sequences similar to simple telomeric repeats. In: Molecular and Cellular Biology 13 (3), 1993: 1583-1589. PMC 359470 (free full text)
- ↑ Harald Biessmann, James M Mason: Telomere maintenance without telomerase. In: Chromosoma 106, 1997: 63-69.
- ↑ James M Mason, Thomas A Randall, Radmila Capkova Frydrychova: Telomerase lost? In: Chromosoma 125 (1), 2016: 65-73. doi: 10.1007 / s00412-015-0528-7 .
- ↑ Mary-Lou Pardue: Drosophila telomeres: Another way to end it all. In: Elizabeth H Blackburn, Carol W Greider (eds.): Telomeres. CSH Laboratory Press, Cold Spring Harbor, New York 1995: 339-370. → "But only the portion of the RNA template encoding the telomeric repeat is copied in the case of telomerase, while the entire RNA transposition intermediate is copied in the case of the retroposons."
- ^ Hermann Joseph Muller: The remaking of chromosomes. In: The Collecting Net, Woods Hole 13, 1938: 181-195, 198.
- ↑ Barbara McClintock: The behavior in successive nuclear divisions of chromosomes broken at meiosis. In: Proc Nat Acad Sci USA 25, 1939: 405-416. PDF . There p. 414: "a normal chromosome 9 [of Zea mays ] with a large terminal knob on the short arm ..."
- ^ Hermann Joseph Muller: An analysis of the process of structural change in chromosomes of Drosophila. In: Journal of Genetics 40, 1940: 1-66 doi: 10.1007 / BF02982481 ; there p. 21: “telomere”. first PDF ; second PDF .
- ↑ Barbara McClintock: The stability of broken ends of chromosomes in Zea mays. In: Genetics 26, 1941, 234-282.
- ↑ Rigomar Rieger, Arnd Michaelis, Melvin M Green: A glossary of genetics and cytogenetics: Classical and molecular. Springer: Heidelberg, Berlin, New York 1968, p. 428 there.
- ^ AW Murray, Jack W Szostak: Construction of artificial chromosomes in yeast. In: Nature ”305 (5931), 1983: 189-193.
- ^ Carol W Greider: Telomeres, telomerase and senescence. In: BioEssays 12 (8), 1990: 357-402. doi: 10.1002 / bies.950120803 .
- ↑ Carol W Greider, Elizabeth H Blackburn: Telomeres, telomerase and cancer. In: Scientific American 274 (2), 1996: 92-97.
- ↑ Elizabeth H Blackburn, Carol W Greider, Jack W Szostak: Telomeres and telomerase: The path from maize, Tetrahymena and yeast to human cancer and aging. In: Nature Medicine 12 (10), 2006: 1133-1138.
- ↑ Elizabeth H Blackburn, ES Epel, J Lin: Human telomere biology: A contributory and interactive factor in aging, disease risk, and protection. In: Science 350 (6265), 2015: 1193-1198. doi: 10.1126 / science.aab3389
- ^ Carlos López-Otín, Maria A. Blasco, Linda Partridge, Manuel Serrano, Guido Kroemer: The Hallmarks of Aging . In: Cell . tape 153 , no. 6 , June 2013, p. 1194–1217 , doi : 10.1016 / j.cell.2013.05.039 , PMID 23746838 , PMC 3836174 (free full text) - ( elsevier.com [accessed May 7, 2019]).
- ↑ Martin Winkelheide : Genetics. The social status can be read from the chromosome ends. Deutschlandfunk . Research Current , April 7, 2014. Primary source: PNAS .
- ↑ Dean Ornish, Jue Lin, June M Chan, Elissa Epel, Colleen Kemp: Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study . In: The Lancet Oncology . tape 14 , no. 11 , October 2013, p. 1112–1120 , doi : 10.1016 / S1470-2045 (13) 70366-8 ( elsevier.com [accessed December 26, 2019]).
- ↑ Julia Merlot: Scott Kelly on the ISS: A twin rarely mutates alone . In: Spiegel Online , March 16, 2018, accessed on March 19, 2018.
- ↑ Danielle M. Townsley, Bogdan Dumitriu, Delong Liu, Angélique Biancotto, Barbara Weinstein, Christina Chen, Nathan Hardy, Andrew D. Mihalek, Shilpa Lingala, Yun Ju Kim, Jianhua Yao, Elizabeth Jones, Bernadette R. Gochuico, Theo Heller, Colin O. Wu, Rodrigo T. Calado, Phillip Scheinberg, Neal S. Young: Danazol Treatment for Telomere Diseases . New England Journal of Medicine 2016; Volume 374, Issue 20 of March 19, 2016, pages 1922-1931; doi: 10.1056 / NEJMoa1515319
- ↑ Peter M Lansdorp: Telomeres on Steroids - Tuning back the mitotic clock? . New England Journal of Medicine 2016; Volume 374, Issue 20 of March 19, 2016, pages 1978-1980; doi: 10.1056 / NEJMe1602822
- ↑ p. 244 ff.