Battery (electrical engineering)
A battery is a store for electrical energy on an electrochemical basis. Batteries that can be recharged, battery , short battery or secondary battery called. Non-rechargeable batteries are called primary batteries, or just batteries .
term
The term " battery " is taken from the military language, where it means a combination of several guns , analogously, an interconnection of several galvanic cells was assigned this term. From the second half of the 20th century, the use of the term “battery” extended to individual primary or secondary cells, whereby the term “accumulator cell” or “accumulator” for short is used for the latter. The change in linguistic usage described was reflected in the DIN standard 40729 accumulators; Galvanic secondary elements; Basic terms addressed, which under battery meant “always several connected cells”, although this terminology has become blurred in the everyday “distinction”.
history
In 1780, the Italian doctor Luigi Galvani noticed that a frog's leg that came in contact with copper and iron kept twitching, and thought it was an electrical effect. The first functioning galvanic element and thus the first battery was invented by Alessandro Volta in the form of the Voltaic column in 1800 . This was followed in the following years design improvements such as the trough battery of William Cruickshank , which avoided the drawback of the vertical structure of the voltaic pile. Historically, a distinction is made between dry batteries - with solid or gel-like electrolyte - and wet batteries - with liquid electrolyte - which are no longer in use today.
The historical wet batteries, which can only be operated in certain positions, include the Daniell element by John Frederic Daniell from 1836 and the different variations and designs in the form of the Gravity Daniell elements , the chromic acid element by Johann Christian Poggendorff from 1842, the Grove element by William Grove from 1844 and the Leclanché element by Georges Leclanché from 1866. These galvanic wet cells were primarily used to power the wired telegraph stations . Over several development steps, the Leclanché element resulted in the still common and position-independent dry batteries, the first work on this came from Carl Gassner , who patented the dry battery in 1887. In 1901, Paul Schmidt first used dry batteries in flashlights in Berlin .
Ancient vessel arrangements interpreted as batteries such as the “ Baghdad battery ” could have generated an electrical voltage of around 0.8 V through the interaction of copper , iron and acid . Whether these vessels were used as batteries in today's sense at the time around 2,000 years ago is controversial and could not be proven beyond doubt.
Basics
An electric cell is an electrochemical energy storage and an energy converter . During the discharge, stored chemical energy is converted into electrical energy by the electrochemical redox reaction . This can be used by an electrical consumer that is independent of the power grid . Alternatively, it can also be used in a consumer that is dependent on the power grid to bridge brief power failures and thus ensure an uninterruptible power supply .
Primary cells can only be discharged once and cannot be recharged. In these cells, the reactions are partially reversible during discharge, but this does not lead to the restoration of an energy content similar to the new state. On the other hand, the rechargeable secondary batteries ( accumulators ) have to be brought to a state of charge similar to the new state, so that a multiple conversion of chemical into electrical energy and back is possible.
The electrode materials determine the nominal voltage of the cell, the amount of materials the energy contained .
Important terms relating to the electrical properties of a battery cell are:
- capacity
- The electrical charge stored in a galvanic cell is called capacitance , which should not be confused with electrical capacitance . The capacity of a battery is given in terms of the electrical charge in ampere-hours ( unit symbol : Ah), or more rarely in ampere-seconds (As) or coulombs (C; 1 As corresponds to 1 C).
- power
- The performance of a battery / battery cell is the amount of electrical energy that can be drawn per unit of time. It is usually given in watts (W). It is the product of the discharge current and discharge voltage or the quotient of energy and time . The energy is the opportunity to work ; it is given in joules (J).
- Energy content
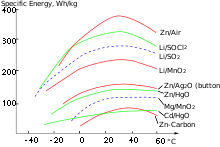
- The energy stored in a battery ( electrical work ) is not always specified, but the energy per mass or per volume is a typical parameter of battery systems and is often listed in the manufacturer's data sheets. An assessment of the type is provided by diagrams of comparative energy data (see diagram). The physical unit is the watt second . The specific energy unit is Ws / m³ or Ws / kg (corresponds to J / m³ or J / kg).
- Self-discharge
- All galvanic cells are subject to self-discharge during storage . The speed of self-discharge depends, among other things, on the type of battery and the temperature. The lower the storage temperature, the lower the self-discharge.
The weakest cell determines the quality of a battery. When connected in parallel , compensating currents lead to greater losses if the cell with a lower capacity is discharged earlier. In series the voltage drops under load together rather because stronger, more charged cell current drive by weaker, already discharged cells where then drops already some of the tension. According to Ohm's law, their increased internal resistance leads to cell heating without the electrical energy being usable.
Areas of application
Depending on the area of application, there are the following terms and assignments:
- "Device batteries" are used to power small, mostly portable devices, for example in flashlights or cell phones . They usually consist of several cells in standardized sizes, both as a single-use primary cell and as a rechargeable battery . Particularly small versions such. B. for hearing aids or quartz watches are called button cells .
- Starter batteries for motor vehicles, drive batteries (traction batteries) or deep cycle storage batteries for electric vehicles and stationary, stationary applications such as uninterruptible power supplies . These batteries are always accumulators .
Type variations
Due to the diverse areas of application with very different requirements in terms of voltage, power and capacity, there are now many types of batteries. These are differentiated, for example
- according to chemical quality in the underlying chemical redox reaction
- Interconnection of cells
- according to cell sizes
Commercially available batteries and cells differ both in their electrical values and in their geometrical or structural shape. Several of the terms listed below can together describe a cell type, e.g. B. "Alkali-Manganese-Cell - LR 6 / AM-3 - AA - Mignon". Often, however, only a certain characteristic is required, e.g. B. the size "AA" for a specially designed flashlight.
According to chemical composition
In the experimental area and to illustrate the functional principle, quite exotic galvanic cells, such as lemon cells, are used.
- See also: accumulator (different accumulator technologies) or supercapacitor (energy storage of another kind).
Primary cells
Galvanic cells that cannot be recharged after discharging are called primary cells or primary elements. The different types are named according to the materials used (with the exception of rechargeable alkaline cells - these are still counted as primary cells):
- Standard batteries (based on zinc-manganese )
- Alkaline manganese battery ; 1.5 V nominal voltage per cell
- Zinc chloride battery ; 1.5 V per cell (almost completely replaced by an alkaline manganese battery)
- Zinc-carbon battery ; 1.5 V per cell (almost completely replaced by alkaline manganese batteries in Europe, apart from some larger types such as lantern batteries )
- Special batteries
- Zinc-air battery ; 1.4 V per cell
- Mercury oxide-zinc battery ; 1.35 V per cell
- Silver oxide-zinc battery ; 1.55 V per cell
- Nickel oxyhydroxide battery ; 1.7 V per cell
- Lithium batteries ; 1.8 V (FeS 2 ) to 3.7 V (SOCl 2 ) depending on the cathode material
- Lithium iron sulfide battery ; 1.5 V per cell
- Aluminum-air battery ; 1.2 V per cell
- Biobattery based on magnesium / NaCl / iron + molybdenum + tungsten, decomposable in the body
- Historic batteries
- Edison Lalande Element ; 0.75 V per element
According to size / design
The most popular batteries for the consumer market
Batteries are often referred to as device batteries, which are very common for powering small electrical appliances such as clocks , radios , toys , flashlights and the like. Ä. and can also be used in permanently installed devices such as smoke detectors .
Device batteries have to be compact, usable in any position, light and yet mechanically robust. With normal storage and use in the device, they must neither leak nor outgas. They are commercially available in a large number of versions based on zinc-carbon or alkali-manganese . Zinc-carbon batteries have been offered less and less since the 2000s and are hardly manufactured today.
There are many types standardized by the IEC and some names from ANSI as well as unofficial names, especially for the nine most common categories.
With the IEC-60086 designations
- R for round , i.e. cylindrical cells,
- F for flat , i.e. a flat design,
- S for square , i.e. an angular design.
These letters can be preceded by another letter to identify the batteries according to their chemical composition:
| Size designation | Dimensions | nominal voltage | Illustration | ||
|---|---|---|---|---|---|
| IEC | ANSI | unofficially | |||
| R20 | 13 | D mono |
Ø 34.2 mm × approx. 61.5 mm | 1.5 V at R20 ( zinc-carbon )
1.5 V for LR20 ( alkali manganese ) |

|
| R14 | 14th | C baby |
Ø 26.2 mm × 50.0 mm
(Ø 25 mm × 49 mm) |
1.5 V for R14 (zinc-carbon)
1.5 V for LR14 (alkali manganese) |

|
| R22C429 | - | Sub-C | Ø 22.2 mm × 42.9 mm
(Ø 22.2 mm × 42.5 mm) |
1.2 V for KR22C429 ( NiCd battery)
1.2 V for HR22C429 ( NiMH battery) |

|
| R12 | - | B. | Ø 21.5 mm × 60.0 mm | 1.5 V for R12 (zinc-carbon)
1.5 V with LR12 (alkali-manganese) |

|
| R23 | A. | approx. Ø 17 mm × 50 mm
(Ø 16.5 mm × 48.5 mm) |
1.2 V as a battery |

|
|
| R6 | 15th | AA mignon |
Ø 14.5 mm × 50.5 mm
(Ø 14.5 mm × 49.5 mm) |
1.5 V at R6 (zinc-carbon)
1.5 V for LR6 (alkali manganese) |

|
| R03 | 24 | AAA micro |
Ø 10.5 mm × 44.5 mm | 1.5 V for R03 (zinc-carbon)
1.5 V for LR03 (alkali manganese) |

|
| R8D425 | 25th | AAAA mini |
Ø 8.3 mm × 42.5 mm | 1.5 V for R8D425 (zinc-carbon)
1.5 V for LR8D425 (alkali manganese) |

|
| R1 | 910 | N L20 Lady |
Ø 12.0 mm × 30.2 mm | 1.5 V at R1 (zinc-carbon)
1.5 V for LR1 (alkali manganese) |

|
| 2R10 | - | Duplex | approx. Ø 21.5 mm × 74 mm | 3.0 V for 2R10 (zinc-carbon) |

|
| 4R44 | - | 4AG13 , L1325 | approx. Ø 13 mm × 25 mm | 6.0 V for 4LR44 (alkali manganese) |

|
| 8R932 | 1811A | 23A | Ø 10.3 mm × 28.5 mm | 12.0 V for 8LR932 (alkaline manganese) |

|
| CR17345 | 5018LC | CR123A | Ø 17 mm × 34.5 mm | 3 V (lithium) |

|
| 3R12 | - | Flat battery | approx. 65 mm × 61 mm × 21 mm | 4.5 V for 3R12 (zinc-carbon)
4.5 V for 3LR12 (alkali manganese) |

|
| 4R25 | 908 915 |
Lantern lantern battery |
approx. 114 mm × 66 mm × 66 mm | 6.0 V for 4R25 (zinc-carbon)
6.0 V for 4LR25 (alkali manganese) |

|
| 6F22 | 1604 | 9 volt block | approx. 48 mm × 26 mm × 17 mm | 9.0 V at 6F22 (zinc-carbon)
9.0 V for 6LR61 (alkali manganese) |

|
| 15F20 | - | 15F20 battery | approx. 50 mm × 25 mm × 15 mm | 22.5 V at 15F20 (zinc-carbon) |

|
Round cells
history
As already mentioned above, a distinction was made until around 1950:
- galvanic cell : concrete combination of electrodes and electrolyte;
- galvanic element : ready-to-use cell enclosed in a container;
- (galvanic) battery : interconnection of several cells to form a unit.
Galvanic elements and batteries were called galvanic power generators for short and were standardized in VDE 0807.
The differences were reflected in the naming of galvanic power generators. This consisted of two or three letters, the nominal voltage and, if applicable, the number of cells:
- first letter: E for element, B for battery;
- second letter: A to R depending on the geometric shape of the cell (cylindrical or cuboid), X or Y for wet elements to differentiate the size (because there is no differentiation between cell and element);
- third letter: for the design of the cell ( T dry cell , F full brown stone , L dry air oxygen);
- in the case of batteries with two or more cells connected in parallel , their number was placed in front of the cell identifier.
Examples:
- ELF : Element from cell L (cylindrical with 50 mm diameter) made of brown stone
- BD 90 : Battery made of cells D (cylindrical with 19.6 mm diameter) with 90 V nominal voltage
- B 2 J 4,5 : Battery made up of two cells J connected in parallel (cylindrical with a 31.5 mm diameter) with a nominal voltage of 4.5 V
- BH 4.5 : Flat battery (30 × 87 × 95 mm) with 4.5 V nominal voltage
Adapter and contact
Not every type of battery is available everywhere. That is why there are, for example, flat battery adapters that accept three AA cells of 1.5 V each. These can be used in all devices that can also accommodate a 4.5 V flat battery (3R12). These adapters are also useful because there are no rechargeable flat batteries.
Small batteries are contacted with spring contacts, more reliable versions are gold-plated. Permanently installed accumulators are provided with plug contacts, screw connections, pole bolts or soldering lugs.
Prefabricated rechargeable batteries, so-called battery packs , consist of several cells that are firmly connected to one another and are often provided with a casing or a housing. In the case of starter batteries , the cells are connected to one another with lead bars, in the case of traction batteries they are usually contacted with copper connectors .
In 2010 Microsoft offered a purely mechanical solution for a battery compartment called " InstaLoad " that allows individual cells to be inserted in any orientation. The contacts are designed in such a way that polarity reversal cannot occur. However, the process did not become relevant.
disposal
Batteries and accumulators do not belong in the residual waste or in the environment, as they contain environmentally harmful and reusable raw materials, which make battery recycling economically attractive for companies. Leaking batteries should also be handled with care, as there are sometimes corrosive substances on the contacts. They also have to be recycled and are considered problematic substances.
In Germany, the Battery Ordinance regulates the return and disposal of batteries. Among other things, it stipulates that no batteries or cells with a mercury content of more than 0.0005 percent by weight may be placed on the market in Germany. In button cell of the mercury content must not exceed 2.0 weight percent. Alkaline-manganese batteries have not contained any mercury since the early 1990s. Before that it was used to amalgamate the electrode material zinc . The poles of lithium batteries must be masked off before disposal.
Small batteries can be returned to retail stores in Germany if they also sell batteries. It is only legally mandatory to take back battery types that the respective retailer carries in the range; however, “foreign” types are usually also accepted, as this does not incur any disadvantages or costs for the dealer. For this purpose, collection containers must be set up there. There is a deposit system for starter batteries in Germany .
Batteries containing harmful substances are also provided with chemical symbols.
- Pb: Battery contains more than 0.004 mass fraction of lead
- Cd: Battery contains more than 0.002 mass fraction of cadmium
- Hg: Battery contains more than 0.0005 mass fraction of mercury
literature
- Lucien F. Trueb, Paul Rüetschi: Batteries and accumulators - Mobile energy sources for today and tomorrow. Springer, Berlin 1998, ISBN 3-540-62997-1 .
- David Linden, Thomas B. Reddy (Eds.): Handbook of Batteries. 3. Edition. McGraw-Hill, New York 2002, ISBN 0-07-135978-8 (English).
- Clive DS Tuck (Ed.): Modern Battery Technology. Ellis Horwood, New York 1991, ISBN 0-13-590266-5 (English).
- Philipp Brückmann: Autonomous Power Supply - Design and Practice of Power Supply Systems with Battery Storage. Ökobuch, Staufen 2007, ISBN 978-3-936896-28-2 .
- Werner Döring : Introduction to Theoretical Physics, Volume II. Berlin, Göschen 1965 (especially the chapter on batteries).
- Michael Sterner , Ingo Stadler (ed.): Energy storage. Need, technologies, integration. 2nd edition, Berlin Heidelberg 2017, ISBN 978-3-662-48893-5 .
Web links
- Advice on batteries and rechargeable batteries - Federal Environment Agency, extensive information brochure (PDF; 3.4 MB)
- History of electrical energy storage
- History of the electric batteries
- Battery Chemistry FAQ (English)
- Foundation for a joint take-back system for batteries
- Technical data and comparison lists for button cells and batteries (PDF; 195 kB)
- Battery Act of June 30, 2009 (PDF; 112 kB)
- General information on rechargeable batteries and batteries (disposal) (PDF; 82 kB)
- Capacity measurements of branded Mignon batteries with different currents (2010)
- Current test reports and comparisons of standard rechargeable batteries and batteries
Individual evidence
- ↑ What is the difference between a battery and an accumulator? In: zvei.org. Retrieved October 18, 2019 .
- ↑ Dirk Flottmann, Detlev Forst, Helmut Roßwag: Chemistry for engineers: Basics and practical examples. Springer, 2003, ISBN 3-540-06513-X , p. 225.
- ^ A b William Edward Ayrton: Practical Electricity . Cassell, London 1891, p. 212 and following . ( Online ).
- ↑ Patent US373064 : Galvanic Battery. Published on November 15, 1887 , inventor: Carl Gassner.
- ↑ Biobattery dissolves in the body . In: orf.at . ORF.at. March 26, 2014. Archived from the original on March 26, 2014.
- ↑ a b Establishing harmonized methods to determine the capacity of all portable and automotive batteries and rules for the use of a label indicating the capacity of these batteries. (PDF; 3.04 MB) In: europa.eu. European Commission DG Environment , September 2008, accessed on March 15, 2012 .
- ↑ a b c INTERNATIONAL STANDARD - IEC 60086-1. (PDF; 529 kB) In: cnlumos.com. International Electrotechnical Commission, December 2006, archived from the original on November 25, 2011 ; accessed on March 15, 2012 (English).
- ↑ a b INTERNATIONAL STANDARD - IEC 60086-2. (PDF; 521 kB) (No longer available online.) In: sztxr.com. International Electrotechnical Commission, 2006, formerly in the original ; accessed on March 15, 2012 (English). ( Page no longer available , search in web archives )
- ^ A b c d Wilhelm Friedrich, Carl Schaub, Gottfried Voltz: Book of tables for electrical engineering (edition C) . For teaching technical knowledge, technical calculation and technical drawing in vocational, craft and technical schools, as well as for self-teaching and practical use for electrical installers and electrical engineers. 175th to 199th edition. Ferd. Dümmlers Verlag, Bonn 1949.
- ↑ InstaLoad Battery Holders ( English ) In: batteryholders.com . Memory Protection Devices Inc. 2016. Archived from the original on March 17, 2016 .: "InstaLoad battery holders are an ingenious solution to guarantee correct battery insertions by users"
- ↑ Systemadmin_Environment: Advice: Batteries and rechargeable batteries . Federal Environmental Agency, May 18, 2013 ( umweltbundesamt.de [accessed on December 4, 2018]).
- ↑ Notes on battery disposal . In: holzkern.com . Time for Nature GmbH.