Covellin
| Covellin | |
|---|---|
| Peacock blue shimmering Covellin from the "East Colusa Mine" near Butte (Montana, USA) | |
| General and classification | |
| other names |
|
| chemical formula | CuS |
|
Mineral class (and possibly department) |
Sulfides and sulfosalts |
|
System no. to Strunz and to Dana |
2.CA.05a ( 8th edition : II / B.15) 08/02/12/01 |
| Similar minerals | Bornite , chalcosine , chalcopyrite |
| Crystallographic Data | |
| Crystal system | hexagonal |
| Crystal class ; symbol | dihexagonal-dipyramidal; 6 / m 2 / m 2 / m |
| Space group | P 6 3 / mmc (No. 194) |
| Lattice parameters | a = 3.79 Å ; c = 16.34 Å |
| Formula units | Z = 6 |
| Frequent crystal faces | {0001}, {10 1 1}, less often {10 1 3}, {10 1 4} |
| Twinning | no |
| Physical Properties | |
| Mohs hardness | 1.5 to 2 (VHN 100 = 128–138) |
| Density (g / cm 3 ) | measured: 4.6 to 4.76; calculated: 4.602 |
| Cleavage | completely after {0001} |
| Break ; Tenacity | uneven; flexible in thin leaflets |
| colour | indigo blue to blue black |
| Line color | gray to black |
| transparency | opaque, translucent in thin leaflets |
| shine | semi-metallic |
| Crystal optics | |
| Refractive indices |
n ω = 1.450 n ε = 2.620 |
| Birefringence | δ = 1.170 |
| Optical character | uniaxial positive |
| Pleochroism | visible: blue to blue-white |
| Other properties | |
| Chemical behavior | soluble in nitric acid |
Covellin , outdated also known as copper indig or blue copper glass as well as its chemical name copper (II) sulfide , is a frequently occurring mineral from the mineral class of " sulfides and sulfosalts ". It crystallizes in the hexagonal crystal system with the chemical composition CuS, so it consists of equal parts of copper and sulfur .
Covellin is generally opaque and only translucent in very thin split leaves . It rarely develops well-developed, tabular to leafy crystals up to 10 cm in size. It is mostly found in the form of fine-grain crusts or powdery hints of other minerals. Fresh covellin samples are characteristic, indigo blue to blue-black in color and have a greasy to semi-metallic sheen . In the air the mineral turns blackish or brightly colored after a while and becomes matt.
Etymology and history
The mineral was first described in 1815 by Johann Carl Freileben as "copper ore of excellent dark glaze, indig or blackish blue color, which is a little lighter and fresher on the quarry than on the surface, where it soon becomes blackish", the one nearby von Sangerhausen was discovered in the "Carolinenschacht" ( towards morning ) partly in the form of thick slabs and layers between layers in copper slate . Free life suspected a new type of mineral behind the blue copper glass and classified it between colored copper ore ( chalcopyrite ) and copper glass ( chalcocene ), but also mentioned that Klaproth regards it as a variety of colored copper ore.
In 1818 August Breithaupt quoted the discovery of Freiesleben in his work Handbuch der Mineralogie , but stated that the mineral was not just a tarnished colored copper ore or glass, as suspected, but was blue in color and non-metallic. He therefore called the mineral copper indigo because of its characteristic color , but without determining its chemical composition.
The exact chemical composition was not determined until 1827 by Nicola Covelli (also Niccolò Covelli , 1790–1829) using the material from Vesuvius and Friedrich August Walchner (1799–1865) using samples from the Haus Baden mine ( Badenweiler , Baden-Württemberg).
Its name, which is still valid today, Covellin, was finally laid down in 1832 by François Sulpice Beudant , who named the mineral after the Italian mineralogist Nicola Covelli ( Niccolò Covelli ; 1790–1829).
classification
Already in the 8th edition of the mineral classification according to Strunz , Covellin belonged to the class of "sulfides and sulfosalts" and there to the department of "sulfides with the molar ratio of metal (M): sulfur (S) = 1: 1", where he was named under the section Other types of structures as named the "Covellin series" with the system no. II / B.15 and the other members Idait , Klockmannit and Valleriit as well as in the appendix with Vulcanite .
In the Lapis mineral directory according to Stefan Weiß, which, out of consideration for private collectors and institutional collections, is still based on this classic system of Karl Hugo Strunz , the mineral was given the system and mineral number. II / C.22-10 . In the "Lapis system" this corresponds to the section "Sulphides with metal: S, Se, Te ≈ 1: 1", where Covellin forms an independent but unnamed group together with Erazoite , Idaite, Klockmannite and Nukundamite (status 2018).
The 9th edition of Strunz's mineral systematics , which has been valid since 2001 and updated by the International Mineralogical Association (IMA) until 2009, also classifies the Covellin in the division of "Metal sulfides, M: S = 1: 1 (and similar)". However, this is further subdivided according to the predominant metals in the compound, so that the mineral can be found according to its composition in the sub-section "with copper (Cu)", where it is the only member of the unnamed group 2.CA.05a .
The systematics of minerals according to Dana , which is mainly used in the English-speaking world , assigns the Covellin to the class of "sulfides and sulfosalts" and there in the department of "sulfide minerals". Here he is together with Klockmannit in the " Covelling group " with the system no. 02.08.12 within the subsection "Sulphides - including selenides and tellurides - with the composition A m B n X p , with (m + n): p = 1: 1".
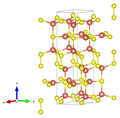
Crystal structure
Covellin crystallizes isotypically with Klockmannite in the hexagonal crystal system in the space group P 6 3 / mmc (space group no. 194) with the lattice parameters a = 3.79 Å and c = 16.34 Å and six formula units per unit cell .
Compared to other compounds, whose relationship formula is also AB and which usually crystallize in the closest packing of spheres , Covellin is built much more complicated. Of the six present in the unit cell are four sulfur atoms, similar to the sulfur atoms in pyrite or patronite when two disulfide anions (S 2 2 ) having a bond length of d (S-S) = 2.07 Å before. The remaining two sulfur atoms are isolated sulfide anions (S 2− ). There are also two different groups of copper cations. Four of the cations are singly charged (Cu + ) and surrounded by sulfur in a tetrahedral manner . One corner of the tetrahedron is made up of a single sulfide anion and the remaining three corners are made up of sulfur atoms that are part of a disulfide anion. The two other copper atoms are doubly charged (Cu 2+ ) and trigonal- planar surrounded by three individual sulfur anions.
| Crystal structure of Covellin |
|
|
| Color table: __ Cu __ S |
properties
With a Mohs hardness of 1.5 to 2, Covellin is one of the soft minerals that, like the reference mineral gypsum (2), can be scratched with the fingernail. Due to its layered structure, the mineral can also be split very easily into thin, flexible leaves.
Covellin has unusual optical properties. Due to a high dispersion , the color of the mineral changes significantly, depending on the medium in which it is located. The dry mineral appears blue in the air; if it is wetted with water, it appears purple. If you immerse it in an oil that is highly refractive, Covellin finally appears red.
Covellin melts easily in front of the soldering tube and burns with a blue flame. It is easily soluble in nitric acid , whereby elemental sulfur is deposited.
Covellin becomes a superconductor below 1.63 Kelvin . Covellin is the first known natural mineral to show this effect.
Modifications and varieties
A total of three varieties of Covellin are known. The blue Covellin , which was first described by Paul Ramdohr in 1931 , contains an excess of copper (68 instead of 66 percent by weight copper). This leads to a significant change in the optical properties. The variety stays blue in water and oil and does not change color.
Two other varieties contain silver and selenium in addition to copper and sulfur .
Education and Locations
Covellin is a typical secondary mineral and is formed by weathering together with other secondary minerals such as anilite , bornite , chalcosine , digigite and djurleit in the so-called cementation zone (below the oxidation zone ) in the area of the water table or just below it. The output minerals pyrite (FeS 2 ) and chalcopyrite (CuFeS 2 ) the resulting already in the oxidation zone, as well as in solution continuous Chalkanthit (CuSO 4 · 5 H 2 O) are converted according to the following reaction equations:
Due to its formation conditions, Covellin is often found as a thin layer on top of other minerals. This type of covellin coating is found in many copper mineral deposits. Thick and ore-rich oxidation and cementation zones occur above all where the water table is subject to large fluctuations. Arid and tropical-arid climate zones therefore offer particularly favorable educational conditions.
Covellin is also rarely formed as a primary mineral under hydrothermal conditions.
In the event of persistent weathering, Covellin itself becomes the starting mineral for the formation of azurite and malachite , as well as an inhomogeneous mineral mixture known as copper pechers.
Covellin is common, but mostly only available in small amounts. An important site and at the same time the type locality is Vesuvius in Italy , where it was created as a sublimation product . Larger crystals were found in Alghero , Sardinia . Other finds with larger amounts of Covellin include in the Mansfeld copper shale near Sangerhausen, in the stratified Lower Silesian copper deposits around Lubin in Poland, near Leogang in Austria, Bor in Serbia, the US states of Montana , Alaska , Colorado and Utah and La Rioja in Argentina been made.
use
Covellin is a copper ore, but since there are hardly any rich deposits, mining only plays a role as a raw material for copper production as a by-product in the mining of other ores.
See also
literature
- Friedrich Klockmann : Klockmann's textbook of mineralogy . Ed .: Paul Ramdohr , Hugo Strunz . 16th edition. Enke, Stuttgart 1978, ISBN 3-432-82986-8 , pp. 448 (first edition: 1891).
- Hans Jürgen Rösler : Textbook of Mineralogy . 4th revised and expanded edition. German publishing house for basic industry (VEB), Leipzig 1987, ISBN 3-342-00288-3 , p. 299 .
Web links
- Mineral Atlas: Covellin (Wiki)
- Covellite search results. In: rruff.info. Database of Raman spectroscopy, X-ray diffraction and chemistry of minerals (RRUFF), accessed on October 13, 2019 .
- American-Mineralogist-Crystal-Structure-Database - Covellite. In: rruff.geo.arizona.edu. Retrieved October 13, 2019 .
Individual evidence
- ^ David Barthelmy: Covellite Mineral Data. In: webmineral.com. Retrieved October 13, 2019 .
- ^ A b c Hugo Strunz , Ernest H. Nickel : Strunz Mineralogical Tables. Chemical-structural Mineral Classification System . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 75 (English).
- ^ Helmut Schrätze , Karl-Ludwig Weiner : Mineralogie. A textbook on a systematic basis . de Gruyter, Berlin; New York 1981, ISBN 3-11-006823-0 , pp. 223-224 .
- ↑ a b c d Covellite . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 (English, handbookofmineralogy.org [PDF; 60 kB ; accessed on October 13, 2019]).
- ↑ a b Covellite. In: mindat.org. Hudson Institute of Mineralogy, accessed October 13, 2019 .
- ↑ Petr Korbel, Milan Novák: Mineral Encyclopedia (= Dörfler Natur ). Edition Dörfler im Nebel-Verlag, Eggolsheim 2002, ISBN 978-3-89555-076-8 , p. 38 .
- ^ Johann Carl Freiesleben : Geognostic contribution to the knowledge of the copper slate mountains, with special reference to a part of the county of Mansfeld and Thuringia . tape 3 . Graz and Gerlach, Freiberg 1815, p. 129–130 ( limited preview in Google Book Search [accessed October 13, 2019]).
- ^ Christian August Siegfried Hoffmann, August Breithaupt: Kupferindig . In: Handbook of Mineralogy . 2nd Edition. tape 4 . Graz and Gerlach, Freiberg 1818, p. 179–180 ( limited preview in Google Book Search).
- ↑ Marco E. Ciriotti, Lorenza Fascio, Marco Pasero: Italian Type Minerals . 1st edition. Edizioni Plus - Università di Pisa, Pisa 2009, ISBN 978-88-8492-592-3 , p. 93 .
- ↑ Thomas Witzke : Discovery of Covellin. In: www.strahl.org. April 23, 2018, accessed October 13, 2019 .
- ^ FS Beudant: Traité Élémentaire de Minéralogie . 2nd Edition. Verdière, Paris 1832, p. 409–410 (French, rruff.info [PDF; 163 kB ; accessed on October 13, 2019] Section “Appendice”: Sulfure de cuivre du Vésuve ).
- ↑ Stefan Weiß: The large Lapis mineral directory. All minerals from A - Z and their properties. Status 03/2018 . 7th, completely revised and supplemented edition. Weise, Munich 2018, ISBN 978-3-921656-83-9 .
- ↑ Ernest H. Nickel, Monte C. Nichols: IMA / CNMNC List of Minerals 2009. (PDF 1703 kB) In: cnmnc.main.jp. IMA / CNMNC, January 2009, accessed October 13, 2019 .
- ^ Howard T. Evans, Judith A. Konnert: Crystal structure refinement of covellite . In: American Mineralogist . tape 61 , 1976, p. 996–1000 (English, minsocam.org [PDF; 577 kB ; accessed on October 13, 2019]).
- ↑ Francesco Di Benedetto, Miria Borgheresi, Andrea Caneschi, Guillaume Chastanet, Curzio Cipriani, Dante Gatteschi, Giovanni Pratesi, Maurizio Romanelli, Roberta Sessoli: First evidence of natural superconductivity: covellite . In: European Journal of Mineralogy . tape 18 , no. 3 , 2006, p. 283–287 , doi : 10.1127 / 0935-1221 / 2006 / 0018-0283 (English, PDF file available online at researchgate.net [accessed October 13, 2019]).
- ↑ Kurt v. Gehlen, Horst Piller: On the look of Covellin . In: Contributions to mineralogy and petrography . tape 10 , 1964, pp. 94-110 , doi : 10.1007 / BF01192539 .
- ^ Argentian Covellite. In: mindat.org. Hudson Institute of Mineralogy, accessed October 13, 2019 . and Selenian Covellite. In: mindat.org. Hudson Institute of Mineralogy, accessed October 13, 2019 .
- ↑ a b Martin Okrusch, Siegfried Matthes: Mineralogie. An introduction to special mineralogy, petrology and geology . 7th, completely revised and updated edition. Springer, Berlin [a. a.] 2005, ISBN 3-540-23812-3 , pp. 32, 36, 37, 282 and 283 .
- ↑ Find location list for Covellin / Covellite in the Mineralienatlas and Mindat , accessed on October 13, 2019.