Gas sensor
A gas sensor is a chemical sensor for the detection of gaseous substances. The proportion of certain chemical constituents of the gas is from the sensor is converted into an electrical signal.
Areas of application and economic importance
Areas of application and example applications
Due to falling prices and increasing safety awareness, gas sensors are spreading in mass markets. Gas sensors can be found in almost every newly built automobile and in almost every household.
- Safety technology: explosion protection ( methane and carbon monoxide detection in mines, hydrogen detection in fuel cells ), detection of gas leaks ( natural gas supply , liquid gas ), poisoning protection (personal carbon monoxide and hydrogen sulfide monitoring), leakage detection (monitoring of volatile organic components in chemical warehouses , Solvents and coolants ), fire detectors (fire gas detection by intelligent detectors), drug test (breath alcohol test for road traffic), detection of chem. Warfare agents ( explosives , poison gas ). Use in gas protection units of ATMs .
- Emission measurement (engine control via lambda probe , vehicle diagnosis , measurement of gaseous immissions in urban traffic)
- Comfort: indoor air quality (automatic ventilation flap in cars, building management, extractor hood)
- Quality assurance: leak detection ( solvent detection ), process monitoring ( lambda probe )
Economical meaning
There are different priorities worldwide. The carbon monoxide -type detection is very common in the US and increasingly in Europe, while in Asia gas sensors primarily for the detection of natural gas in household and heating systems are used. The gas sensor in automobile construction for the automatic control of the air circulation flap of the interior ventilation was initially only found in Europe, now worldwide. The determination of air quality in industrial security technology and building management is mainly common in North America, Africa and Europe, and increasingly also in Asia. In the security technology of public facilities, the area of application is mainly the USA. The global market for gas sensor and gas analysis systems is estimated at around 1.5 billion dollars per year, with Germany ranking third in the list of world market leaders behind the USA and Japan in the manufacture of sensors.
Requirements for gas sensors
- high selectivity: restriction of the sensor reaction to a target substance
- high sensitivity: gas detection in the desired concentration interval, either a few percent by volume up to a few ppm
- Stability: chemical, electrical, mechanical
- long service life: 10 years and more
- for fast applications: small thermal and chemical time constants
- low power requirement
- easy calibration capability with the greatest possible time between the required calibrations
- low specimen variance
- easy handling
- low price
Depending on the application, these requirements are weighted differently.
Measurement principles and modes of operation
Problems with chemical sensors
The field of chemical sensors is relatively young. While sensors for measuring physical parameters such as temperature , pressure and acceleration are sealed watertight and airtight, a chemical sensor such as the gas sensor interacts directly with its environment. As a result, it is also much more susceptible to poisoning (environmental influences that make the sensor insensitive), cross-sensitivity (substances other than the target component that cause a sensor signal), corrosion , drift and aging.
Overview of measuring principles
There are many options for gas detection that differ in the way chemical information is converted into electronic information. Ultimately, there must always be an electronic signal that can be processed by the downstream electronics. But until that happens, a u. U. multi-layered implementation using different physical quantities and sensor principles, a selection of which is presented here. Depending on the application (which gas is to be detected? What concentrations are to be expected? How quickly must the measurement take place? What environmental conditions is the gas sensor exposed to? How expensive can the sensor be? What is its commercial availability? What maintenance intervals are permitted? Etc. ) a suitable sensor principle must be selected.
Physical measurement methods
Use of molecular properties for detection: molecular mass , diffusion behavior , molecular structure (magnetic properties, e.g. paramagnetism in the oxygen sensor ), molecular stability ( binding energy ) and molecular mobility.
Chemical measurement methods
Utilization of chemical properties such as reactivity , oxidisability and reducibility .
Electrical principles
Resistive, chemo-resistor
With resistive principles, the gas or gas mixture to be measured directly influences the conductivity of a gas-sensitive sensor layer. This change in resistance serves as a measured variable. Examples:
- inorganic metal oxide semiconductor (MOX)
- organic phthalocyanine
- conductive polymer
Capacitive
The measured variable here is the capacitance of a capacitor , which is influenced by a gas-sensitive dielectric . Examples:
- Polymer sensors for moisture measurement
Potentiometric
With the potentiometric sensor, the sensor itself generates a voltage that can be measured directly. Examples:
- Solid-state ion conductor ( lambda probe )
- Chemo transistor (polymer field effect transistor )
Amperometric
An amperometric sensor delivers a measurable current . Examples:
- electrochemical cell ( Clark electrode , fuel cell )
- Flame ionization detector
- Photoionization detector
Thermal
In the case of thermal principles, the temperature increase due to a chemical reaction on the sensor surface is measured or the thermal conductivity of the gas is used directly as a measured variable.
Thermochemical
Chemical reactions take place on the sensor surface in which energy is given off in the form of heat. This increase in temperature is measured. Example:
- Catalytic Converter (e.g. Pellistor)
Thermal-physical
Direct measurement of the thermal conductivity of the gas atmosphere. Example:
Gravimetric
With gravimetric sensors, a change in mass is measured. Gas molecules, for example, are deposited on the surface of an oscillating crystal and thereby change its resonance frequency. Such sensors work on the principle of a piezoelectric sensor . Examples:
Optically
Optical gas sensors are also based on physical principles in which the optical properties of a sample space filled with gas are characterized. Examples:
- Refractive index
- Absorption spectrum in the infrared range, IR spectroscopy
- Intensity , luminescence
Biochemically
Biochemical gas sensors follow the biological model of the implementation of certain substances or groups of substances. You use several of the principles listed above to convert the signal. See main article biosensor .
Functioning of common sensor principles
Catalytic effect
Working principle
In catalytic bead sensors , the sensor effect is created by the combustion of adsorbed gases on the surface of a catalyst . Activation energy is necessary for a chemical reaction to take place . The catalyst has the effect of reducing this activation energy. Intermediate states of the reaction form on its surface, which would not be possible without it. Gas molecules are separated (homolytic dissociation ) and are available to the reaction partners on the catalyst surface . This process takes place in every vehicle catalytic converter . For example, a starting material ( nitrogen monoxide , NO) reacts to form two reaction products ( oxygen and nitrogen ). With gas sensors, however, two starting materials usually react to form only one product, for example carbon monoxide and oxygen to form carbon dioxide . The catalyst itself is ideally not involved in the reaction and remains unchanged. Catalyst materials are, for example, noble metals such as platinum (Pt) or palladium (Pd), but also metal oxides such as manganese oxide or copper (II) oxide .
The catalyst has no influence on the energy balance of a reaction, but only influences the speed at which the reaction takes place. The rate at which the starting materials react to form the reaction product depends on the concentration of the reactants and the temperature.
The reaction only takes place on the catalyst surface at catalytically active surface sites (active centers). The adsorption of the gaseous substance is exothermic, i.e. with the release of heat energy and the desorption is endothermic (absorption of heat energy from the environment). If the catalyst is offered a substance that no longer desorbs from the surface because there is insufficient desorption energy available, the catalyst is poisoned by this substance because the active centers are blocked. Catalyst poisons are, for example, sulfur compounds ( SO 2 , H 2 S ) or silicone-containing substances from oils or cleaning agents (e.g. hexamethyldisiloxane (HMDS, plasticizers in cable insulation)).
If, during a chemical reaction, a particle changes from a state with a higher energy to a state of lower energy, the energy difference can be given off in the form of heat. In the static case, this results in a temperature increase in the system. I.e. the measurement of the temperature can therefore be used to determine the reaction rate, which in turn depends on the concentration of the reactants present. This effect is used in so-called pellistors by measuring the heat tint caused by the change in resistance of a platinum wire due to the increased temperature. The heating wire also serves as a temperature sensor ( resistance thermometer ). Flammable gases (e.g. methane , propane , butane , hydrogen ) can be detected using pellistors . Non-combustible gases are i. A. not recognized because the effect is too small. The Pellistor is therefore primarily suitable for high gas concentrations in the lower percentage range and is often used to monitor limit values ( explosion limit ), for example in explosion protection.
Designs
The classic design of a pellistor is a resistance heater in the form of a wound platinum wire embedded in a ceramic bead. This pearl is coated with a catalytically active substance. The heat of reaction penetrates through the surface of the bead to the platinum wire. As it heats up, it changes its resistance, which is used as a measured variable. See explosimeter . In order to reduce the power consumption required for heating, work is being carried out on manufacturing pellistors also micromechanically using silicon technology. Here a silicon chip is processed in such a way that a very thin (a few micrometers) membrane remains as the carrier for the sensor layer. The main advantages of this membrane construction are: Low power consumption, since less energy is required to reach the operating temperature due to the thin membrane (i.e. high thermal resistance ). Mass production in a batch process .
advantages
- robust
- inexpensive
- linear sensor response as a function of the gas concentration
- relatively easy to calibrate (one-point calibration )
disadvantage
- low sensitivity in the percentage range, since relatively high gas concentrations are necessary for a sufficiently high heat release.
- Low selectivity: Every gas that burns on the pellistor's catalyst surface and causes a measurable heat release is registered by the pellistor as an increase in resistance, so that a selective determination of the type of gas is difficult.
- relatively high power requirement (1–4 W)
Metal oxide semiconductor gas sensors (MOX)
Working principle
Certain metal oxides such as tin (IV) oxide (SnO 2 ) change their conductivity under the influence of gas. Other materials are zinc oxide , titanium dioxide or organic semiconductor materials such as MePTCDI . In tin dioxide sensors, oxygen vacancies in the crystal lattice act like n- doping of the material. This effect is based on metal oxide semiconductor gas sensors (MOX). Oxygen molecules from the ambient air adsorb on the sensor surface. This creates oxygen ions, which in turn can irreversibly react with flammable gases. For example, if the molecules of a reducing gas hit the surface of the semiconductor, they react with the adsorbed oxygen. One example is the reaction of carbon monoxide to carbon dioxide . Due to the energetic position of the adsorption sites on the tin dioxide surface (in the band model below the Fermi level ), the oxygen takes electrons from the inside of the semiconductor and is therefore negatively charged. In the case of an n-type semiconductor (SnO 2 ), this reduces the charge carrier density, a depletion zone of electrons is formed and the conductivity in the edge zone is reduced. In the band model one speaks of a band bending.
The energetic position of the reaction product is above the Fermi level and not occupied, as a result of which an electron is given back to the semiconductor, the reaction product is desorbed and the conductivity increases again. An equilibrium is created between adsorption and desorption of oxygen, carbon monoxide and carbon dioxide. This leads to a change in the oxygen coverage of the surface of the sensor, which changes the amount of band bending (energy barrier) and thus the conductivity of the sensor, which can be measured macroscopically as a change in resistance.
Reducing gases such as B. carbon monoxide and hydrogen cause an increase in conductivity in the n-HL, while oxidizing gases reduce the conductivity. The amount of change in conductivity depends on the gas concentration.
The above consideration applies to single-crystal HL material. As described, the increase or decrease in conductivity affects the edge zone, the grain boundary of the crystal . In order to increase the sensitivity, many of these grain boundaries are required, which is why one tries in the sensor production to give the HL material as polycrystalline as possible . In order for as many gas molecules as possible to reach the grain boundaries, the surface should also be as porous as possible. The electron flow through the sensor is then dominated by the energy barrier at the grain boundaries. The height of the energy barrier in turn depends on the gas concentration, so that the following experimental relationship results:
G: conductance, G 0 : basic conductance, c: gas concentration, r: empirically determined exponent
However, this relationship is extremely simplified and only applies to a gas under controlled laboratory conditions. The complex chemical reaction mechanisms that take place on the sensor are still the subject of current research.
Designs
Two technologies are most commonly used to manufacture sensitive layers: thin-film and thick-film technology . In thin-film technology, layer thicknesses in the range from 10 nm to 5 µm are common, which are applied by physical or chemical processes such as thermal vapor deposition , sputtering or chemical vapor deposition . Thick film technology refers to coating processes with which layers between 10 µm and 80 µm are produced. An example of this is the screen printing process , in which a paste-like mass is applied to the sensor carrier material ( substrate ) using a doctor blade through a template . This manufacturing method is used to create layers that are as porous as possible. The porosity increases the surface of the sensor material, which means that more gas reaches the grain boundaries and thereby increases the sensitivity. Just as there are different methods for producing the sensor layer, there are different technologies for the sensor substrate. Traditionally, a ceramic substrate is used on which the gas-sensitive metal oxide layer is applied. In comparison, there are also MOX sensors with a microstructured structure using Si technology with a membrane structure. The ceramic substrate is several hundred µm thick and, compared to the approximately 5000 times thinner Si membrane, requires a significantly higher heating power (≈ 2 W for ceramic substrates, ≈ 80 mW for membrane structures). The membrane mostly consists of silicon dioxide or silicon nitride and has a low heat capacity and a high heat resistance . As a result, the heating power introduced by the heater practically only heats the membrane, while the chip frame remains almost at ambient temperature. The structure in silicon technology thus reduces the required heating power and enables mass production in a batch process .
- The Taguchi sensor
A well-known sensor is the “Figaro sensor” based on tin oxide developed by Naoyoshi Taguchi. Type TGS 813 is used in particular to detect natural gas, methane gas, type TGS 822 is used to detect alcohol, ammonia, etc. As early as 1988, for example, 400,000 of the TGS 813 sensors (good detection for natural gas and methane) were sold.
The sensitivity of the Taguchi sensor depends crucially on the average grain size of the tin oxide and this should not exceed a grain size of 10 nm. In very dry air, the sensitivity of the TGS 822 to ethanol is very limited. The change in voltage drops sharply. In the 30–60% humidity range, the TGS 822 reacts to ethanol changes in humidity, but only very marginally. If only one gas is present, the relative concentration can be determined by calibration.
advantages
- inexpensive through mass production
- high sensitivity in the ppm range
- long service life (depending on the design (classic, micromechanical))
disadvantage
- Non-linear sensor response as a function of the gas concentration
- high drift and aging (also depending on the design: classic, micromechanical)
- Calibration difficult due to non-linearity
- Cross-sensitivities e.g. B. Humidity
- low selectivity
- high energy consumption due to the required operating temperature (1–4 W) (reduced by miniaturization and thin-film technology )
Infrared optical gas sensors (NDIR)
Working principle
NDIR gas sensors use the different absorption of infrared radiation by gases to measure the gas concentration in a similar way to absorption spectrometry . The abbreviation NDIR stands for non-dispersive infra-red. In contrast to spectrometry, the spectral range of interest is not scanned point by point ( dispersive ). Rather, a sum signal is formed in a single step.
Absorption of infrared radiation by gases
The absorption of radiation in the case of gases takes place in the infrared wavelength range mainly through the excitation of vibrations and rotation of the gas molecules. Quantum physics provides the explanation . The necessary energies result from the distances between the energy levels of the quantum mechanical ground states ( ) and the excited states ( ). However, transitions between higher levels are also possible. In the first case then photons of energy can
be absorbed. Here h and c are natural constants ( Planck's quantum of action and speed of light ) and λ the wavelength of the radiation. However, further conditions must be met. So transitions are only allowed between energetically neighboring states. In order for electromagnetic radiation to interact with the gas at all, the dipole moment of the molecule to be excited has to change during absorption.
The latter is not possible with all gases. In the case of noble gases , for example, no oscillations or rotations can be excited because their molecules only consist of a single atom. Elementary diatomic molecules such as oxygen or nitrogen also do not absorb in the infrared spectral range, since they do not have a permanent dipole moment and only vibrations that do not generate a dipole moment are possible with them. However, the existence of a permanent dipole moment is not a prerequisite for radiation absorption. The carbon dioxide molecule does not have a permanent dipole moment, but it absorbs infrared radiation very well. The symmetrical stretching vibrations do not cause any change in the dipole moment and therefore also no absorption. This is caused by the excitation of buckling and asymmetrical stretching vibrations.
NDIR measuring principle
With the NDIR principle, the optical transmission of the gas is measured in a selected spectral range. This is determined by means of a bandpass filter in such a way that it encloses a sufficiently intense and characteristic absorption band of the gas as precisely as possible . The illustration shows this using the example of carbon dioxide.
From this, the concentration of the gas can be calculated using Lambert-Beer's law :
This is the transmission of the gas, α the absorption coefficient, c the concentration of the gas and d the length of the absorption path. In gas measuring devices, the path length is usually the inner length of the gas cuvette, i.e. the distance between the optical cuvette windows. The conditions and limits for the validity of the law must be observed. For example, there are deviations at high concentrations, because here the interaction of the gas molecules can no longer be neglected, as is assumed by Lambert-Beer’s law. In practice, a basic structure as shown in the illustration of the transmission measurement arrangement is usually used.
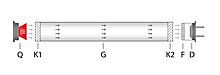
In it, Q is an infrared radiation source, K1 and K2 are the windows of the gas cuvette with the gas G, F is the optical bandpass filter and D is a broadband infrared detector. So-called thermal detectors are mostly used as detectors. These first convert the radiation via a broadband absorbing layer into a temperature increase of a temperature-sensitive element such as a pyroelectric chip or a thermopile . The latter then generates the sensor signal via a further conversion and possibly an amplification. For professional gas measuring sections, pyroelectric detectors are mostly used due to their higher detectivity.
The signal voltage is then the spectral sum of all products from the specific radiation of the source (S (λ)), the sensitivity of the detector (E (λ)), the detector area (A) and the transmissions of all elements in between :
For the calculation of the signal, it is sufficient if the beginning and end of the integration area ( and ) are defined in such a way that all areas are covered that could contribute to the signal.
The quotient from the signal with gas and without gas is usually used to eliminate the constants. The latter can be implemented in a multi-channel detector by means of a reference channel in which a band-pass filter is used, in the passage range of which there are no absorption bands of the gases occurring in the mixture to be measured. In practical use, the gas measuring module is often calibrated with the aid of test gas mixtures.
Designs
The designs of NDIR gas measurement modules are essentially determined by the design of the gas cuvette . The simplest variant of the cuvette is a tube with a radiation source and a detector with a bandpass filter at the ends. The length of the cuvette is a few millimeters to a few tens of centimeters.
Smaller designs can be achieved through a folded beam path using reflectors. With all plug-in modules, the emitter and detector are usually arranged on one side and a reflector on the opposite side. The dimensions are a few centimeters.
If long path lengths are required, for example in the case of weakly absorbing gases, the aforementioned constructions can no longer be used. Cells with multiple reflections are then used, for example, Pfund, White, Herriott or similar cells.
advantages
NDIR gas detectors are simple, robust and inexpensive. With a clocked radiation source, you can do without any mechanically moving parts. The principle allows a simple function check when using an additional reference channel. If the concentration of a few known gases in a gas mixture is to be measured from a few 10 ppm to 100% with medium resolution, the method is particularly suitable. It is therefore particularly suitable for portable or stationary industrial measuring devices and gas warning devices.
disadvantage
The method can only be used with infrared active gases. Noble gases and elementary diatomic gases (such as oxygen or nitrogen) cannot be measured. Furthermore, the absorption bands of the gas to be measured must be known in order to be able to select the appropriate bandpass filter. A gas measuring module is therefore always restricted to the gases for which it was developed. Weakly absorbing gases require long path lengths and thus either long cuvettes or complicated multiple reflection cells. Low concentrations of weakly absorbing gases and high concentrations of strongly absorbing gases cannot be measured with the same cuvette. Very low concentrations in the lower ppm or ppb range cannot be measured. Due to the exponential relationship in Lambert-Beer's law, the measurement resolution is also subject to such, that is, the measurement resolution deteriorates with increasing concentration. If a large number of gases have to be measured, the method is unsuitable because a channel would have to be used for each gas. Even gases with closely adjacent or overlapping absorption bands can only be distinguished with great effort. However, such gases can often be separated by measuring in the area of a second absorption band. However, this requires an additional measuring channel.
Operating modes and increase in selectivity
Most of the operating principles of gas sensors are usually very broadband, i. H. the sensor reacts in the same way to a large number of different substances in the environment. However, the aim of the manufacturers is to produce sensors that are as selective as possible.
Sensor layer optimization
One possibility to generate selectivity is the targeted optimization of the sensor layer for a target component or group of substances, for example by adding catalyst materials.
filter
Another method to increase selectivity is to use filters. These are placed in the sensor housing in front of the sensitive layer and filter unwanted components ( cross-sensitivity ).
Operating modes
Single sensor, constant operation
The operating temperature of catalytic or MOX sensors is between 200 ° C and 500 ° C. A single sensor is operated in this way when the measurement problem is simple and, for example, only a group of gases is to be detected (e.g. alarm threshold of combustible gases with a pellistor). The signal evaluation and electronic wiring is often a measuring bridge . To compensate for the influence of the ambient temperature and humidity, two individual sensors are usually housed in a housing, one of which has been passivated .
In the case of more complex problems, such as the analysis of gas mixtures or the detection of a target gas in front of a dominant interference background, a significantly higher effort is required for intelligent operating modes and signal evaluation with multivariate analysis methods ( main component analysis , discriminant analysis , artificial neural network , electronic nose ).
Sensor array
If different types of sensors are interconnected to form a sensor field, the individual sensors have different sensitivities for a specific gas. Characteristic signal patterns are thus generated. However, these arrays have the disadvantage that they are expensive, the sensors age differently and are therefore subject to different drift phenomena.
Virtual multisensor
The chemical reactions that take place on the surface of a gas sensor depend on the temperature, so that the properties of a gas sensor also depend on the operating temperature. It is therefore advisable to operate a single sensor at different operating temperatures. A suitable temperature modulation (temperature cycle, T-cycle) turns a single sensor into a virtual multi-sensor , which, depending on the operating temperature, shows different behavior for the same gas supply and thus enables signal evaluation using multivariate analysis methods . A distinction is made between continuous temperature cycles (e.g. sinusoidal) and discrete. Discrete temperature cycles allow rapid evaluation in the seconds range and enable pulsed operation, i.e. H. Breaks between the individual cycles, which is energy-saving and an important criterion for battery-operated systems. Such short T-cycles are only made possible by micromechanically structured substrates with a membrane structure. The time constant for thermal settling to the target temperature is around 20 ms for such membrane sensors, while it is several seconds for sensors with a thick ceramic substrate. Because of the microstructuring of the sensors and the resulting small thermal mass (membrane), cycle times from 50 ° C to 400 ° C in ten temperature levels are possible in a few seconds. This means that gas detection is very fast, which is of crucial importance for early warning systems, for example. When designing temperature cycles, it must be ensured that the sensor response of HL gas sensors in the event of a temperature jump in the presence of reducing or oxidizing gases consists of two overlapping effects. On the one hand the reaction of the sensitive layer to the temperature change and on the other hand the reaction of the layer to the actual gas supply and the setting of a new state of equilibrium on the sensor surface. The chemical reaction at one temperature level runs i. A. much slower than the rapid thermal settling, whereby the minimum stage duration is determined by the chemical reaction.
Recent developments
A new approach to sensors is based on so-called microcantilevers . These are tiny tips like those used in atomic force microscopes . They are coated with a material that specifically binds the analyte in question. Cantilevers can vibrate like a spring. If additional analyte molecules are bound, the mass of the microcantilever changes and thus the frequency with which it oscillates and which is recorded as a measured variable. A research group coated cantilever made of silicon having a three-dimensionally ordered layer of titanium dioxide - nanotubes . Titanium dioxide can bind substances that contain nitro groups well, which z. B. is characteristic of TNT and other explosives. About 500,000 of the nanotubes can be accommodated on a cantilever. The sensor was still able to detect concentrations of TNT in air of less than one ppt within 3 minutes. A practical use of these sensors for a selective detection system for explosives or other gases is still pending.
Another method is based on so-called gold mesoflowers, gold particles approx. 4 µm in size that are coated with silicon dioxide and serve as a carrier for tiny silver clusters . These are embedded in a protein ( albumin ). Irradiated with light of a suitable wavelength, the silver clusters luminesce red. The gold increases the fluorescence. If a solution containing TNT is applied, it reacts with the amino groups of the albumin to form a Meisenheimer complex . This will extinguish the red glow of the silver clusters. A TNT concentration of 1 ppb already extinguishes the glow. By combining it with surface -enhanced Raman scattering ( SERS, surface-enhanced Raman scattering ), detection limits down to the zeptomole range (10 −21 mol ) can be achieved.
Individual evidence
- ↑ a b Isatron: gas detector | Isatron , accessed August 14, 2018
- ↑ a b c d e Hanno Schaumburg: Sensor applications . Springer-Verlag, 2013, ISBN 978-3-322-96721-3 , pp. 334 ( limited preview in Google Book search).
- ↑ a b c Gerhard Wiegleb: Industrial gas sensors, measuring methods - signal processing - application technology - test criteria; with 17 tables . expert verlag, 2001, ISBN 978-3-8169-1956-8 , p. 77 ( limited preview in Google Book search).
- ↑ a b c d e f g h i j Ghenadii Korotcenkov: Handbook of Gas Sensor Materials Properties, Advantages and Shortcomings for Applications Volume 2: New Trends and Technologies . Springer Science & Business Media, 2013, ISBN 978-1-4614-7388-6 , pp. 124,158,197,249,365 ( limited preview in Google Book search).
- ^ A b c d Hans-Rolf Tränkler, Ernst Obermeier: Sensor technology manual for practice and science . Springer-Verlag, 2013, ISBN 978-3-662-09866-0 , pp. 1116 ( limited preview in Google Book search).
- ↑ Jörg Hoffmann: Taschenbuch der Messtechnik . Carl Hanser Verlag GmbH Co KG, 2015, ISBN 978-3-446-44511-6 , pp. 290 ( limited preview in Google Book Search).
- ↑ Hans-Rolf Tränkler, Leonhard M. Reindl: Sensor technology manual for practice and science . Springer-Verlag, 2015, ISBN 978-3-642-29942-1 , pp. 1118 ( limited preview in Google Book search).
- ↑ a b c d Gerhard Wiegleb: Gas measurement technology in theory and practice Measurement devices, sensors, applications . Springer-Verlag, 2016, ISBN 978-3-658-10687-4 , pp. 431 f., 691 ( limited preview in Google Book search).
- ^ A b Georg Schwedt, Torsten C. Schmidt, Oliver J. Schmitz: Analytical Chemistry Basics, Methods and Practice . John Wiley & Sons, 2016, ISBN 978-3-527-69877-6 ( limited preview in Google Book Search).
- ↑ Google Patents: EP3105571A1 - Process and sensor system for measuring the concentration of gases - Google Patents , accessed on August 15, 2018
- ↑ KIMESSA: Wärmetönung / Pellistor - KIMESSA , accessed on August 15, 2018
- ↑ Infrared detector. www.infratec.de, accessed on January 5, 2018 .
- ↑ a b c Research Center Dresden-Rossendorf: gas sensors
- ^ A b S. Yamauchi: Chemical Sensor Technology . Elsevier, 2012, ISBN 978-0-444-59946-9 , pp. 1 ( limited preview in Google Book search).
- ^ S. Matsuura: New developments and applications of gas sensors in Japan. In: Sensors and Actuators B: Chemical. 13, 1993, p. 7, doi: 10.1016 / 0925-4005 (93) 85311-W .
- ^ A b Jacob Y. Wong, Roy L. Anderson: Non-Dispersive Infrared Gas Measurement . Lulu.com, 2012, ISBN 978-84-615-9732-1 , pp. 26 ( limited preview in Google Book search).
- ↑ Cross-sensitivities when measuring gas in NDIR. (PDF) www.saxon-junkalor.de, January 2013, accessed on January 5, 2018 (pdf, 29 kB).
- ↑ Gas analysis. www.infratec.de, accessed on January 5, 2018 .
- ↑ Elisabetta Comini, Guido Faglia, Giorgio Sberveglieri: Solid State Gas Sensing . Springer Science & Business Media, 2008, ISBN 978-0-387-09665-0 , pp. 305 ( limited preview in Google Book Search).
- ↑ Denis Spitzer, Thomas Cottineau, Nelly Piazzon, Sébastien Josset, Fabien Schnell, Sergei Nikolayevich Pronkin, Elena Romanovna Savinova, Valérie Keller: A bio-inspired nanostructured sensor for the detection of very low explosive concentrations . In: Angewandte Chemie. 124, 2012, pp. 5428-5432, doi: 10.1002 / anie.201108251 .
- ↑ Ammu Mathew, PR Sajanlal, Thalappil Pradeep: Selective Visual Detection of TNT at the Sub-Zeptomole Level. In: Angewandte Chemie. 124, 2012, pp. 9734-9738, doi: 10.1002 / anie.201203810 .
literature
- W. Jessel: Gases-Vapors-Gasmesstechnik. Dräger Safety AG & Co. KGaA self-published, 2001.
- K. Bachkaus, B. Erichson: Multivariate analysis methods. Springer Verlag, Heidelberg 2006
- F. Dickert: Chemosensors for gases and solvent vapors, chemistry in our time, 26th year 1992, No. 3, pp. 139-143, ISSN 0009-2851
- F. Dickert, O. Schuster: Piezoelectric Chemosensoren, Chemistry in Our Time, 28th year 1994, No. 3, pp. 147-152, ISSN 0009-2851
- T. Elbel: Microsensors. Vieweg, 1996.
- J. Gardner: Microsensors - Principles and Applications. Wiley & Son, 1994
- H. Günzler, HU Gremlich: IR Spectroscopy - An Introduction. Wiley-VCH, Weinheim 2003, ISBN 3-527-30801-6 .
- W. v. Münch: Materials in electrical engineering. Teubner Verlag, 1993.
- TC Pearce, SS Schiffmann, HT Nagle, JW Gardner (eds.): Handbook of Machine Olfaction. Electronic nose technology. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 2003.
- H. Schaumburg: sensors. Teubner, 1992, ISBN 3-519-06125-2 .
- H. Schaumburg: Sensor Applications. Teubner, 1995, ISBN 3-519-06147-3 .
- E. Schrüfer, L. Reindl, B. Zagar: Electrical measurement technology - measurement of electrical and non-electrical quantities . Hanser, 2018 (12th edition). ISBN 978-3-446-45698-3 .
- H.-R- Tränkler, LM Reindl (Hrsg.): Sensortechnik - manual for practice and science . Springer Vieweg, 2014 (2nd edition). ISBN 978-3-642-29942-1 . * A. Zell: Simulation of neural networks. Oldenbourg Verlag, 2000, ISBN 3-486-24350-0 .
Web links
- GOSPEL is the European Network of Excellence in Artificial Olfaction
- Karlsruhe Institute of Technology (KIT) - Research center for fire protection technology: KAMINA - Karlsruhe gas sensor microarrays for rapid fire gas analysis
- Advanced Gasmitter (AGM)
- Gasmessage.de: Presentation of measurement methods and further information