Butanone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Butanone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 8 O | |||||||||||||||
| Brief description |
highly flammable, colorless, acetone-like smelling liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 72.11 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.805 g cm −3 |
|||||||||||||||
| Melting point |
−86 ° C |
|||||||||||||||
| boiling point |
79.6 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
Easily soluble in water (292 g l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.3788 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
|
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Butanone (often also methyl ethyl ketone , abbreviated MEK ) is one of the most important industrially used ketones alongside acetone . It is a colorless, easily mobile liquid with a typical odor and is mainly used as a solvent .
nomenclature
Butanone is isomeric to the corresponding aldehyde called butyraldehyde .
Extraction and presentation
2-butanone industrially by dehydrogenation of 2-butanol in the gas phase at temperatures of 400-500 ° C under atmospheric pressure in the presence of zinc or copper oxide catalysts prepared.
One works in the gas phase and uses tube bundle reactors for the dehydrogenation.
In addition, there is also a process in the liquid phase in which the dehydrogenation is carried out at temperatures around 150 ° C. over Raney nickel catalysts .
A biotechnological production from butane-2,3-diol is based on renewable raw materials .
In 2011 around 1.05 million tons per year were used worldwide .
properties
Physical Properties
Butanone is a colorless, low-viscosity liquid with a typical ketone-like odor that is very similar to that of acetone . The compound boils at 79.6 ° C. under normal pressure. According to Antoine, the vapor pressure function results according to log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 3.9894, B = 1150.207 and C = −63.904 . The compound forms azeotropically boiling mixtures with a number of solvents . The azeotropic compositions and boiling points can be found in the following table. No azeotropes are with toluene , m -xylene , ethylbenzene , 1-propanol , n -butanol , iso -butanol , sec -butanol , allyl alcohol , acetone, 1,4-dioxane , methyl acetate , isopropyl acetate , n -butyl acetate , isobutyl acetate , formic acid and Acetic acid formed.
| Azeotropes with various solvents | |||||||||||
| solvent | water | n -hexane | n -heptane | Cyclohexane | benzene | chloroform | Carbon tetrachloride | ||||
| Content of butanone | in% (m / m) | 88.7 | 29.5 | 71.3 | 44.1 | 44.3 | 83 | 29 | |||
| boiling point | in ° C | 73.4 | 64.3 | 77.0 | 71.8 | 78.4 | 80 | 74 | |||
| solvent | Methanol | Ethanol | 2-propanol | tert -butanol | Ethyl acetate | Methyl propionate | Diisopropyl ether | Di- n -propyl ether | |||
| Content of butanone | in% (m / m) | 32.8 | 60.9 | 70.4 | 69.0 | 18.0 | 60.0 | 16.2 | 74.6 | ||
| boiling point | in ° C | 63.9 | 74.0 | 77.5 | 78.7 | 77.0 | 79.0 | 66.8 | 78.3 | ||
The miscibility with water is limited. As the temperature rises, the solubility of butanone in water decreases or the solubility of water in butanone increases.
| Solubilities between butanone and water | ||||||||||||
| temperature | ° C | 0 | 9.6 | 19.3 | 29.7 | 39.6 | 49.7 | 60.6 | 70.2 | |||
| Butanone in water | in% (m / m) | 35.7 | 31.0 | 27.6 | 24.5 | 22.0 | 20.6 | 18.0 | 18.2 | |||
| Water in butanone | in% (m / m) | 10.9 | 11.1 | 11.2 | 11.3 | 11.7 | 11.9 | 13.4 | 13.7 | |||
Important thermodynamic quantities are given in the following table:
| property | Type | Value [unit] | Remarks |
|---|---|---|---|
| Standard enthalpy of formation | Δ f H 0 liquid Δ f H 0 gas |
−273.3 kJ mol −1 −238.1 kJ mol −1 |
as a liquid as a gas |
| Enthalpy of combustion | Δ c H 0 liquid | −2444.2 kJ mol −1 | as a liquid |
| Heat capacity | c p | 158.91 J mol −1 K −1 (25 ° C) 2.204 J g −1 K −1 (25 ° C) |
as a liquid |
| Critical temperature | T c | 535.7 K | |
| Critical pressure | p c | 41.5 bar | |
| Critical density | ρ c | 3.74 mol·l −1 | |
| Enthalpy of fusion | Δ f H | 8.439 kJ mol −1 | at the melting point |
| Enthalpy of evaporation | Δ V H | 31.3 kJ mol −1 | at normal pressure boiling point |
The temperature dependence of the evaporation enthalpy can be calculated according to the equation Δ V H 0 = A e (−βT r ) (1 − T r ) β (Δ V H 0 in kJ / mol, T r = (T / T c ) reduced temperature ) with A = 51.87 kJ / mol, β = 0.2925 and T c = 536.8 K in the temperature range between 298 K and 371 K.
Chemical properties
2-butanone is stable at room temperature and in the absence of atmospheric oxygen. In the presence of atmospheric oxygen, peroxides can be formed during prolonged storage. The targeted oxidation with atmospheric oxygen by means of catalysts leads to diacetyl . The reaction with amyl nitrite leads to oxidation in the α-position to the monooxime of diacetyl.
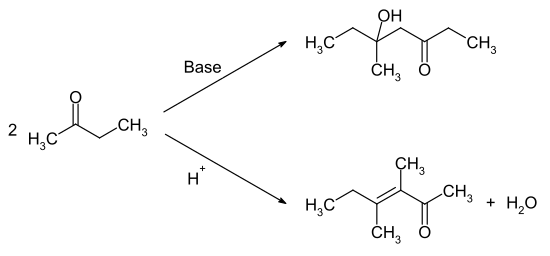
The reaction with hydrogen peroxide gives the methyl ethyl ketone peroxide , which can be used as a polymerization initiator . The reaction with nitric acid or other strong oxidizing agents results in a mixture of formic acid and propionic acid . Self-condensation leads to different reaction products in the basic or acidic medium. Under basic catalysis, the carbonyl function reacts with the methyl group, while under acidic conditions the methylene group α-position attacks the carbonyl function.
The condensation with formaldehyde gives the methyl isopropyl ketone . 2-Butanone forms addition products with hydrogen cyanide and with sodium hydrogen sulfite or potassium hydrogen sulfite . The reaction with hydroxylamine gives the 2-butanone oxime . The halogenation takes place in the α-position to the keto group. Tertiary alcohols are formed with Grignard compounds .
Safety-related parameters
Butanone forms highly flammable vapor-air mixtures. The compound has a flash point of −7.5 ° C. The explosion range is between 1.5% by volume (45 g / m 3 ) as the lower explosion limit (LEL) and 12.6% by volume (378 g / m 3 ) as the upper explosion limit (UEL). With a minimum ignition energy of 0.27 mJ, vapor-air mixtures are extremely ignitable. The maximum explosion pressure is 9.3 bar. The maximum explosion pressure decreases with increasing temperature and reduced outlet pressure. The limit oxygen concentration at 20 ° C is 9.5% by volume. The value tends to increase with decreasing pressure and decrease with increasing temperature. The limit gap width was determined to be 0.85 mm. This results in an assignment to explosion group IIB. The ignition temperature is 475 ° C. The substance therefore falls into temperature class T1. The electrical conductivity of 3.6 · 10 −7 S · m −1 is rather low.
| Maximum explosion pressure and limit oxygen concentration under reduced pressure | ||||||||||||
| pressure | in mbar | 1013 | 600 | 400 | 300 | 200 | 150 | 100 | ||||
| Maximum explosion pressure | in cash | at 20 ° C | 9.5 | 5.7 | 2.7 | 1.8 | 1.4 | 0.9 | ||||
| at 100 ° C | 7.5 | 4.6 | 3.1 | 1.5 | 0.7 | |||||||
| Limit oxygen concentration | in vol% | at 20 ° C | 9.5 | 9.5 | 9.9 | |||||||
| at 100 ° C | 8.5 | 8.5 | 8.7 | 9.1 | 12.5 | |||||||
use
Like acetone, butanone is a good solvent in which a wide range of plastics , resins and paints can be dissolved. It is also used for dewaxing lubricating oils , degreasing metal surfaces, extracting fats and oils from natural resins, as an artificial flavor and for sterilizing medical instruments. The reaction of butanone with hydrogen peroxide produces methyl ethyl ketone peroxide, an important radical initiator for the polymerization of polyester resins . Since 1962 it has been used in Germany as a denaturant for ethanol because of its similar boiling point .
In 2001, 950,000 tons of butanone were used worldwide.
Safety instructions / toxicology
Butanone was included in 2017 by the EU in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation in the Community's ongoing action plan ( CoRAP ). The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. Butanone uptake was caused by concerns about consumer use , environmental exposure, worker exposure , high (aggregated) tonnage, high risk characterization ratio (RCR) and widespread use, as well as the possible risk of reproductive toxicity and as a potential endocrine disruptor . The re-evaluation has been running since 2018 and is carried out by Sweden .
Web links
- International Chemical Safety Card (ICSC) for methyl ethyl ketone at the National Institute for Occupational Safety and Health (NIOSH).
Individual evidence
- ↑ a b c d e f g h i j k l m n o p Entry for CAS no. 78-93-3 in the GESTIS substance database of the IFA , accessed on October 17, 2016(JavaScript required) .
- ↑ a b c d e f g h Entry on butan-2-one. In: Römpp Online . Georg Thieme Verlag, accessed on November 01, 2016.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-74.
- ↑ Entry on Butanone in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limits - Current MAK and BAT values (search for 78-93-3 or 2-butanone ), accessed on September 15, 2019.
- ↑ Butanone data sheet from AlfaAesar, accessed on January 24, 2010 ( PDF )(JavaScript required) .
- ↑ a b c Manfred Baerns, Arno Behr, Axel Brehm, Jürgen Gmehling, Kai-Olaf Hinrichsen, Hanns Hofmann, Regina Palkovits, Ulfert Onken, Albert Renken: Technische Chemie . 2nd Edition. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany 2013, ISBN 978-3-527-33072-0 , p. 599 .
- ^ Roland Dittmeyer, Wilhelm Keim, Gerhard Kreysa, Alfred Oberholz (eds.): Winnacker • Küchler: Chemical technology - processes and products - organic intermediate compounds, polymers . 5th edition. tape 5 . Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 2005, ISBN 978-3-527-30770-8 .
- ↑ a b c d e f g h i j k l m n o p q r Detlef Hoell, Thomas Mensing, Rafael Roggenbuck, Michael Sakuth, Egbert Sperlich, Thomas Urban, Wilhelm Neier, Guenther Strehlke: 2-Butanone. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim 2012; doi: 10.1002 / 14356007.a04_475.pub2 .
- ^ JK Nickerson, KA Kobe, John J. McKetta: The Thermodynamic Properties of the Methyl Ketone Series. In: J. Phys. Chem. 65, 1961, pp. 1037-1043; doi: 10.1021 / j100824a038 .
- ↑ Entry on 2-butanone (phase change data). In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD, accessed November 17, 2019.
- ↑ a b c I. M. Smallwood: Handbook of organic solvent properties. Arnold, London 1996, ISBN 0-340-64578-4 , pp. 175-177.
- ^ A b R. M. Stephenson: Mutual Solubilities: Water-Ketones, Water-Ethers, and Water-Gasoline-Alcohols. In: J. Chem. Eng. Data . 37, 1992, pp. 80-95; doi: 10.1021 / je00005a024 .
- ↑ a b c d e G. C. Sinke, FL Oetting: The Chemical Thermodynamic Properties of Methyl Ethyl Ketone. In: J. Phys. Chem. 68, 1964, pp. 1354-1358; doi: 10.1021 / j100788a014 .
- ↑ a b c W. B. Kay, CL Young: Gas-liquid critical properties. Diethylamine, 2-butanone (methylethyl ketone) system. In: Int. DATA Ser., Sel. Data Mixtures. Ser. A, 1976, no. 1, p. 76.
- ^ A b V. Majer, V. Svoboda: Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation. Blackwell Scientific Publications, Oxford 1985, p. 300.
- ↑ a b c d e E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ↑ BG RCI-Merkblatt T 033 Avoidance of ignition hazards due to electrostatic charges. Jedermann-Verlag, 2009, ISBN 978-3-86825-103-6 .
- ↑ a b c D. Pawel, E. Brandes: Final report on the research project, the dependence of safety parameters on the pressure below atmospheric pressure. ( Memento of December 2, 2013 in the Internet Archive ), Physikalisch-Technische Bundesanstalt (PTB), Braunschweig 1998.
- ↑ BAuA Federal Institute for Occupational Safety and Health: TRGS 727: Avoidance of ignition hazards due to electrostatic charges. BAuA Federal Institute for Occupational Safety and Health, July 29, 2016, accessed on July 6, 2018 (German).
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Butanone , accessed on March 26, 2019.