Phototrophy
Phototrophy or photrophy (from ancient Greek φῶς phos = the light + τροφή trophé = the diet) describes the use of light as an energy source by living beings . The light is needed to synthesize the energy-rich chemical substance adenosine triphosphate (ATP) as an energy carrier and short-term energy store. With this ATP synthesis, living beings convert light energy into chemical energy.
Only certain organisms can use light energy directly for their metabolism. They are called phototrophic organisms or phototrophs . Phototrophy is common among prokaryotes (living things with cells without a nucleus) as well as among eukaryotes (living things with cells with a nucleus).
The phototrophic prokaryotes are metabolically divided into two groups. The first group uses light energy with the help of chlorophyll pigments ( chlorophylls or bacteriochlorophylls ). The second group is limited to a few archaea . They use light energy with the help of the rhodopsin pigments bacteriorhodopsin , proteorhodopsin or xanthorhodopsin . These are not structurally similar to chlorophyll pigments. In contrast, the phototrophic eukaryotes do not show any such metabolic physiological diversity.
Phototrophy should not be confused with phototropy (color change in crystalline substances and glasses due to exposure to light).
Forms of phototrophy
To perform phototrophy, organisms need very specific dyes ( pigments ). These special pigments are located in biomembranes . There they absorb light and make the radiation energy contained in it usable. So far, two different classes of such pigments have been discovered in phototrophic organisms: chlorophylls (chlorophylls, bacteriochlorophylls) and rhodopsins (bacteriorhodopsin, proteorhodopsin, xanthorhodopsin).
Phototrophy with chlorophylls: light-dependent reaction of photosynthesis
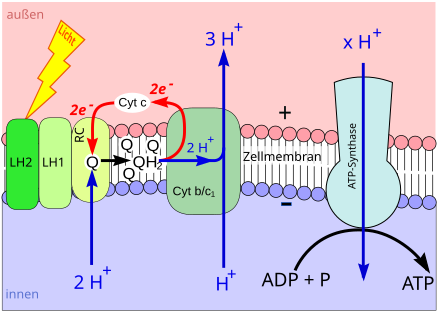
During photosynthesis , chlorophyll (or bacteriochlorophyll) is converted from its basic chemical state into an energetic (“excited”) state by the energy of light. In an excited state, a chlorophyll molecule easily gives off an energetic electron.
The electron is passed on via certain other molecules ( electron transport chain ), which are also located in the biomembrane. In the course of electron transport, hydrogen ions ( protons , H + ) are scooped from one side of the biomembrane to the other. That is why their concentration increases on one side of the membrane and at the same time decreases on the other side. This creates a high H + concentration gradient between the two sides of the membrane ( proton gradient ).
The proton gradient is used to build up ATP: the ATP synthase is embedded in the biomembrane . This is an enzyme , the synthesis of ATP from adenosine diphosphate (ADP) and phosphate (P i ) catalyzes . A channel runs within the ATP synthase that connects both sides of the membrane. Protons flow through the channel along their concentration gradient (→ diffusion ). The kinetic energy of the hydrogen nuclei flowing through is used by the ATP synthase for the synthesis of ATP, i.e. converted into chemical energy (→ chemiosmosis ).
Light energy is used to build up a proton gradient. The proton gradient is used to synthesize ATP. This phototrophic process is part of the so-called light - dependent reaction of photosynthesis.
The connection of P i to other substances is called phosphorylation . During the light-dependent reaction, ADP is phosphorylated to ATP with the help of light. Hence the process is called photophosphorylation .
A distinction is made between different forms of photosynthesis. During oxygenic photosynthesis , water molecules are split. The splitting of the water molecules ( photolysis ) also requires light and chlorophyll. The electron supply for the electron transport chain is obtained from the split water molecules. In addition, oxygen is released. In anoxygenic photosynthesis , other organic or inorganic substances are used for the supply of electrons and not water. No light is required for this and no oxygen is released.
| Electron donation (at) or | Photosynthesis form | Occurrence |
|---|---|---|
| Iron-II-ions (Fe 2+ ) | anoxygenic photosynthesis | Purple bacteria |
| Nitrite (NO 2 - ) | anoxygenic photosynthesis | Purple bacteria |
| elemental sulfur (S 0 ) | anoxygenic photosynthesis | Purple bacteria |
| Hydrogen sulfide (H 2 S) | anoxygenic photosynthesis | green non-sulfur bacteria, green sulfur bacteria, purple bacteria |
| Thiosulfate (S 2 O 3 2− ) | anoxygenic photosynthesis | Purple bacteria |
| Water (H 2 O) | oxygenic photosynthesis | Cyanobacteria, phototrophic eukaryotes |
| Hydrogen (H 2 ) | anoxygenic photosynthesis | green non-sulfur bacteria |
Phototrophy with rhodopsins
The phototrophic ATP synthesis (photophosphorylation) with the help of bacteriorhodopsin , proteorhodopsin or xanthorhodopsin also proceeds chemiosmotically . A rhodopsin consists of a protein that spans an entire biomembrane ( transmembrane protein ). Inside the protein is a molecule called retinal .
If light of a certain wavelength hits the retinal, the molecule changes its shape. In the process, a proton is released to the outside of the biomembrane. A new hydrogen nucleus is then fed to the retinal from the inside of the membrane. With the new proton, the molecule falls back into its original shape - until it is hit by light again, changes its shape again and releases a proton to the outside of the membrane once more. In this way, a rhodopsin-based phototrophy creates a high H + concentration gradient between both sides of the membrane (proton gradient).
The proton gradient is broken down by the fact that protons flow back through the channel of an ATP synthase along their concentration gradient to the inside of the membrane. The kinetic energy of the hydrogen nuclei flowing through is used for the synthesis of ATP (→ function of bacteriorhodopsin ).
Phototrophic organisms
Phototrophic prokaryotes
Different prokaryotes have evolved different forms of phototrophy. On the one hand, ATP synthesis developed with the help of the pigments bacteriorhodopsin, proteorhodopsin or xanthorhodopsin. On the other hand, phototrophy evolved independently with the help of chlorophyll pigments, photosynthesis. For many of the phototrophic prokaryotes, phototrophy is not the only option for energy metabolism. Especially in the dark, you may be able to use different ways of providing chemotrophic energy.
| Prokaryotes | Substance and energy change | Phototrophy form |
|---|---|---|
| Cyanobacteria | Photolithoautotrophy (photohydroautotrophy) | oxygenic photosynthesis |
| green non-sulfur bacteria | Photoorganoheterotrophy or photolithoautotrophy | Type II anoxygenic photosynthesis |
| green sulfur bacteria | Photolithoautotrophy or photoorganoheterotrophy | Type I anoxygenic photosynthesis , even at hydrothermal springs in the deep sea |
| Haloarchaea | Photoorganoheterotrophy | Phototrophy with bacteriorhodopsin |
| Heliobacteria | Photoorganoheterotrophy | Type I anoxygenic photosynthesis |
| Non-sulfur purple bacteria | Photolithoautotrophy or photoorganoheterotrophy | Type II anoxygenic photosynthesis |
| Thermoplasmates | Photoorganoheterotrophy | Phototrophy with proteorhodopsin |
| α-proteobacteria | Photoorganoheterotrophy | anoxygenic photosynthesis of type II or phototrophy with proteorhodopsin |
|
Fulvimarina pelagi
(an α-proteobacterium) |
Photoorganoheterotrophy | Type II anoxygenic photosynthesis and phototrophy with xanthorhodopsin |
| β-proteobacteria | Photoorganoheterotrophy | Type II anoxygenic photosynthesis |
| γ-proteobacteria | Photoorganoheterotrophy | anoxygenic photosynthesis of type II or phototrophy with proteorhodopsin |
| Flavobacteria | Photoorganoheterotrophy | Phototrophy with proteorhodopsin |
| Salinibacter ruber | Photoorganoheterotrophy | Phototrophy with xanthorhodopsin |
| Sulfur purple bacteria | Photolithoautotrophy | Type II anoxygenic photosynthesis |
Phototrophic eukaryotes
Originally, eukaryotes were not phototrophic. However, some eukaryotes acquired the ability to phototrophy by forming communities for mutual benefit ( mutualisms ) with phototrophic organisms. Such mutualisms arose several times, at different times and independently of one another. Plastids represent the most advanced form of phototrophic mutualism (→ endosymbiotic theory ).
Most of the phototrophic eukaryotes practice some form of photolithoautotrophy, which is sometimes called separately as photohydroautotrophy. This means that they synthesize carbohydrates exclusively with light, water and carbon dioxide. These phototrophic eukaryotes carry out oxygenic photosynthesis with the help of their mutualistic partners. Phototrophy is not the only source of nutrients for many phototrophic eukaryotes. They can also feed themselves chemoorganoheterotrophically by supplying themselves with nutrients by completely or partially eating other organisms. Such life forms, both autotrophically and heterotrophically, practice mixotrophy . It was only recognized a few years ago that many organisms that were previously classified as autotrophic or heterotrophic are in reality mixotrophic.
Algae and land plants
The most famous phototrophic eukaryotes harbor phototrophic plastids, the chloroplasts, in their cells. The chloroplast organelle emerged only once and that about 1.6 billion years ago. At that time it was possible to keep a cyanobacterium permanently inside the eukaryotic cell. So-called primary chloroplasts were formed. The primary chloroplasts of red algae and glaucophytes, which are presumably homologous to the other chloroplasts, are (mainly for historical reasons) called rhodoplasts (in red algae) and as muroplasts (in the past also: cyanelles) in glaucophytes.
Other eukaryotes later ingested such eukaryotes together with their primary chloroplasts (or alternatively: rhodoplasts). The ingested eukaryotes were gradually reduced until almost only their primary chloroplasts were left. In this way, secondary, complex chloroplasts were formed . Unlike the primary chloroplasts, these are then surrounded by four cell membranes instead of two. The inner two membranes go back to the chloroplast itself, the third to an enclosing vacuole and the fourth to the original cell membrane of the enclosed organism that contained the chloroplasts. In many cases, no further remnants of this organism are morphologically recognizable, but genes that originate from the host can still be detected, subsequently integrated into the host's core genome.
The uptake of chloroplasts was particularly varied in the Dinophyceae or armored flagellants. In this group, chloroplasts were apparently obtained multiple times and independently of one another. Most armored flagellants have secondary chloroplasts from red algae, but others have chloroplasts derived from recruited green algae. A small group of Dinophyceae even has tertiary chloroplasts. They come from ingested diatoms , gullet or calcareous algae , which in turn possessed secondary chloroplasts.
When the phototrophic eukaryotic cells divide , their chloroplasts multiply in them in parallel. Eukaryotes with such chloroplasts are grouped under the name algae . Most algae are microalgae . They remain microscopic, often live as protozoa or form cell colonies or coenobia with a very limited number of cells. Few groups of algae have developed multicellular forms. Such macroalgae are found exclusively among the Rhodophyta (red algae), the Phaeophyta (brown algae) and the Chloroplastida (green algae and the like).
A group of algae managed to colonize the land permanently; today they form land plants (next to them, often unicellular, microalgae occur as colonies or coatings on land). They belonged to the Streptophytina , which belong to the Charophyta , which in turn are assigned to the Chloroplastida. The chloroplasts of all chloroplastida appear green. The oldest fossils of green land plants are 475 million years old. These are spores of plants that are likely to have belonged to the liverworts . Today's land plants can be divided into mosses and tracheophyta ( vascular plants ).
| large eukaryotic group | phototrophic mutualist |
|---|---|
| Chlorarachniophyta ( green pseudopods ) | complex chloroplasts |
| Chloroplastida : Chlorophyta ( green algae ), Embryophyta ( land plants ) u. a. | Chloroplasts |
| Chrysophyta ( golden algae ): Bacillariophyceae ( diatoms ), Chrysophyceae ( golden algae ), Xanthophyceae ( yellow-green algae ) | complex rhodoplasts |
| Cryptophyta ( gullet ) | complex rhodoplasts |
| Dinophyta ( armored flagellant ) | complex rhodoplasts |
| Euglenophyta ( flagella ) | complex chloroplasts |
| Glaucophyta | Muroplasts (formerly called cyanelles) |
| Haptophyta ( calcareous algae ) | complex rhodoplasts |
| Phaeophyta ( brown algae ) | Phaeoplasts (complex rhodoplasts) |
| Rhodophyta ( red algae ) | Rhodoplasts |
Phototrophic protists without chloroplasts
In addition to the phototrophic protists with chloroplasts, there are many other phototrophic unicellular organisms that do not have chloroplasts. These protists acquire phototrophy in other ways. It often happens by ingesting other phototrophic protozoa - cyanobacteria or zoochlorella.
In addition to photosynthesis based on chlorophyll, primary energy production (not based on endosymbionts) using the rhodopsin pigment proteorhodopsin through DNA sequencing was detected not only in numerous autotrophic, but also a large number of heterotrophic protists, which are actually forms with a mixed diet (mixotrophic ) would have to be classified, the ecological consequences of which have so far been poorly understood. This ability has also passed to autotrophic and, to a few heterotrophic, eukaryotes via symbiogenesis. The (heterotrophic) dinoflagellate Oxyrrhis marina even has numerous rhodopsins from two different families: In addition to the sensory rhodopsins widespread in eukaryotes, proteorhodopsins have also been detected, which drive a proton pump using sunlight and thus contribute to energy production; It has been shown experimentally that the species survives periods of starvation longer when exposed than in darkness. Corresponding proteorhodopsins were later also detected in other autotrophic and heterotrophic dinoflagellates.
| Protist | large eukaryotic group | particular phototrophic mutualist
or other form of phototrophy |
|---|---|---|
| Auranticordis quadriverberis | Cercozoa | Cyanobacteria |
| Large foraminifera | Foraminifera ( chamberlings ) | Zoochlorella or zooxanthellae |
| Hatena arenicola | Cryptophyta ( gullet ) | Nephroselmis (a green algae) |
| Oxyrrhis marina | Dinozoa | Phototrophy with proteorhodopsin |
| Paramecium bursaria | Ciliophora ( eyelash animal ) | Zoochlorella |
| Paulinella chromatophora | Cercozoa | Cyanobacteria ( Prochlorococcus or Synechococcus ) |
| Stentor polymorphus | Ciliophora (eyelash animal) | Zoochlorella |
Phototrophic opisthoconta
Not only algae, land plants and certain protists do phototrophy. Even among the opisthokonta - that is, among fungi and animals - some groups were able to switch to the phototrophic way of life. Phototrophy was achieved in very different ways.
- Phototrophy with photobionts : Lichenes ( lichens ) are mutualisms between fungi and phototrophic protozoa, which are collectively called photobionts . Many photobionts are single- or few-celled chlorophyta (green algae). The remaining photobionts come from the group of cyanobacteria.
- Phototrophy with zoochlorella : Different animals evolved mutualisms between themselves and endosymbiotic green algae of the genus Chlorella ( zoochlorella ). Better known representatives are the green freshwater sponges Ephydatia fluviatilis and Spongilla lacustris . The cnidaria ( cnidarians ) include the green hydra ( Hydra viridissima ), which stores chlorella in vacuoles inside their cells. The cnidaria also include the green giant anemones Anthopleura elegantissima and Anthopleura xanthogrammica . The green giant anemones can not only harbor zoochlorella, but also zooxanthellae.
- Phototrophy with other green algae : In addition to the frequently occurring zoochlorella, other single-celled chlorophyta (green algae) also entered into phototrophic mutualisms with animals. The green alga Tetraselmis convolutae lives between the cells of the green acoelomorph Symsagittifera roscoffensis . And Oophila amblystomatis lives in eggs and embryos of Ambystoma maculatum ( spotted transverse newt ). This newt is the only known example of a phototrophic vertebrate.
- Phototrophy with zooxanthellae : Even more often than zoochlorella, zooxanthellae are used as phototrophic mutualists by animals. The most widespread are zooxanthellae of the genus Symbiodinium , which belong to the armored flagellants . Many cnidarians harbor zooxanthellae, especially the various corals . But zooxanthellae are also found in many sea anemones, for example, in the wake Rose ( Anemonia sulcata ), in the sun rose ( Cereus pedunculatus ) and - in addition zoochlorella - in the green giant anemones Anthopleura elegantissima and Anthopleura xanthogrammica . Zooxanthellae are still found in some jellyfish . For example in the umbrella jellyfish Mastigias papua and Linuche unguiculata and in the mangrove jellyfish Cassiopea xamachana . Outside of the cnidarians, only a few cases of animal mutualisms with zooxanthellae have so far been found. At least they occur in the Acoelomorpha genus Waminoa and in Tridacnidae, the giant clams .
- Phototrophy with kleptoplastids : representatives of the sacoglossa ( gastropod snails ) rob chloroplasts from green algae. You are doing kleptoplasty . To do this, they eat cells from certain green algae. In the intestine, the chloroplasts are removed from the eaten cell contents. They are then transported through the screw body. Finally, the chloroplasts are stored in vacuoles in skin cells. These chloroplasts, which were originally stolen by the eaten green algae, are called klepto (chloro) plast (id) en . The best-known phototrophic representatives of the gastropod snails are the green velvet snail ( Elysia viridis ) and its close relative Elysia chlorotica .
- Phototrophy with xanthopterin : The oriental hornet ( Vespa orientalis ) seems to have evolved a completely new way of using sunlight. To do this, she uses the yellow dye xanthopterin (in conjunction with the brown dye melanin ) in the cuticle of her exoskeleton in order to produce electricity in the same way as a solar cell. The biological purpose of the structure is not yet known. It may be used to generate chemical energy that helps the wasp dig underground nest cavities.
See also
literature
- Donat-Peter Häder (Ed.): Photosynthesis . Stuttgart / New York 1999, ISBN 3-13-115021-1 .
- MT Madigan, JM Martinko: Brock Microbiology . Munich 2006, ISBN 3-8273-7187-2 .
- U. Sonnewald: Physiology. In: Strasburger textbook of botany. Heidelberg 2008, ISBN 978-3-8274-1455-7 .
- J. Overmann, F. Garcia-Pichel: The Phototrophic Way of Life. In: The Prokaryotes, Vol. 2: Ecophysiology and Biochemistry. New York 2006, ISBN 0-387-30742-7 .
Individual evidence
- ^ U. Sonnewald: Physiology. In: Strasburger textbook of botany. Heidelberg 2008, ISBN 978-3-8274-1455-7 , p. 274.
- ↑ MT Madigan, JM Martinko: Brock microbiology . Munich 2006, ISBN 3-8273-7187-2 , pp. 604-617.
- ↑ a b c d M. T. Madigan, JM Martinko: Brock microbiology . Munich 2006, ISBN 3-8273-7187-2 , pp. 613-614.
- ^ BM Griffin, J. Schott, B. Schink: Nitrite, an electron donor for anoxygenic photosynthesis. In: Science. 316, 2007, p. 1870 doi: 10.1126 / science.1139478
- ↑ a b M. T. Madigan, JM Martinko: Brock microbiology . Munich 2006, ISBN 3-8273-7187-2 , p. 621.
- ↑ a b M. T. Madigan, JM Martinko: Brock microbiology . Munich 2006, ISBN 3-8273-7187-2 , p. 456.
- ↑ a b M. T. Madigan, JM Martinko: Brock microbiology . Munich 2006, ISBN 3-8273-7187-2 , pp. 444-447.
- ↑ MT Madigan, JM Martinko: Brock microbiology . Munich 2006, ISBN 3-8273-7187-2 , pp. 448-449.
- ↑ MT Madigan, JM Martinko: Brock microbiology . Munich 2006, ISBN 3-8273-7187-2 , p. 478.
- ↑ AK Sharma, JL Spudich, WF Doolittle: Microbial rhodopsin: functional versatility and genetic mobility. In: Trends in Microbiology. 14, 2006, pp. 463-469 doi: 10.1016 / j.tim.2006.09.006
- ^ A. Martinez, AS Bradley, JR Waldbauer, RE Summons, EF DeLong: Proteorhodopsin photosystem gene expression enables photophosphorylation in a heterologous host. In: PNAS 104, 2007, pp. 5590-5595 doi: 10.1073 / pnas.0611470104
- ↑ DA Bryant, Frigaard NU: Prokaryotic photosynthesis and phototrophy illuminated. In: Trends in Microbiology. 14, 2006, pp. 488-496 doi: 10.1016 / j.tim.2006.09.001
- ↑ MT Madigan, JM Martinko: Brock microbiology . Munich 2006, ISBN 3-8273-7187-2 , p. 465.
- ↑ JT Beatty, J. Overmann, MT Lince, AK Manske, AS Lang, RE Blankenship, Van Dover CL, Martinson TA, Plumley FG: An obligately photosynthetic bacterial anaerobe from a deep-sea hydrothermal vent. In: PNAS. 102, 2005, pp. 9306–9310 doi: 10.1073 / pnas.0503674102 (pdf)
- ↑ MT Madigan, JM Martinko: Brock microbiology . Munich, 2006: 476,478, ISBN 3-8273-7187-2 .
- ↑ MT Madigan, JM Martinko: Brock microbiology . Munich 2006, ISBN 3-8273-7187-2 , p. 431.
- ↑ MT Madigan, JM Martinko: Brock microbiology . Munich 2006, ISBN 3-8273-7187-2 , p. 376.
- ^ E. Duchow, HC Douglas: Rhodomicrobium vannielii, a new photoheterotrophic bacterium. In: Journal of bacteriology. 58, 1949, pp. 409-416 (pdf)
- ^ KL Straub, FA Rainey, F. Widdell: Rhodovulum iodosum sp. nov. and Rhodovulum robiginosum sp. nov., two new marine phototrophic ferrous-iron-oxidizing purple bacteria. In: International Journal of Systematic Bacteriology. 49, 1999, pp. 729-735. PMID 10319496
- ^ NU Frigaard, A. Martinez, TJ Mincer, EF DeLong: Proteorhodopsin lateral gene transfer between marine planktonic Bacteria and Archaea. In: Nature 439, 2006, pp. 847-850 doi: 10.1038 / nature04435
- ^ VV Yurkov, JT Beatty: Aerobic Anoxygenic Phototrophic Bacteria. In: Microbiology and Molecular Biology Reviews. 62, 1998, pp. 695–724 PMC 98932 (free full text)
- ↑ N. Jiao, Y. Zhang, Y. Zeng, N. Hong, R. Liu, F. Chen, P. Wang: Distinct distribution pattern of abundance and diversity of aerobic anoxygenic phototrophic bacteria in the global ocean. In: Environmental Microbiology. 9, 2007, pp. 3091–3099 doi: 10.1111 / j.1462-2920.2007.01419.x (pdf)
- ↑ de la Torre JR, Christianson LM, Béjà O, Suzuki MT, Karl DM, Heidelberg J, DeLong EF: Proteorhodopsin genes are distributed among divergent marine bacterial taxa. In: PNAS. 100 (2003): 12830-12835 doi: 10.1073 / pnas.2133554100
- ↑ JC Cho, SJ Giovannoni: Fulvimarina pelagi gen. Nov., Sp. nov., a marine bacterium that forms a deep evolutionary lineage of descent in the order "Rhizobiales". In: International Journal of Systematic and Evolutionary Microbiology. 53, 2003, pp. 1853-1859 doi: 10.1099 / ijs.0.02644-0
- ↑ I. Kang, HM Oh, SI Lim, S. Ferriera, SJ Giovannoni, JC Cho: Genome Sequence of Fulvimarina pelagi HTCC2506T, a Mn (II) -Oxidizing Alphaproteobacterium Possessing an Aerobic Anoxygenic Photosynthetic Gene Cluster and Xanthorhodopsin. In: Journal of Bacteriology. 192, 2010, pp. 4798-4799 doi: 10.1128 / JB.00761-10
- ↑ N. Jiao, Y. Zhang, Y. Zeng, N. Hong, R. Liu, F. Chen, P. Wang: Distinct distribution pattern of abundance and diversity of aerobic anoxygenic phototrophic bacteria in the global ocean. In: Environmental Microbiology. 9, 2007, pp. 3091-3099. doi: 10.1111 / j.1462-2920.2007.01419.x (pdf)
- ↑ N. Jiao, Y. Zhang, Y. Zeng, N. Hong, R. Liu, F. Chen, P. Wang: Distinct distribution pattern of abundance and diversity of aerobic anoxygenic phototrophic bacteria in the global ocean. In: Environmental Microbiology. 9, 2007, pp. 3091–3099 doi: 10.1111 / j.1462-2920.2007.01419.x (pdf)
- ↑ L. Gómez-Consarnau, N. Akram, K. Lindell, A. Pedersen, R. Neutze, DL Milton, JM González, J. Pinhassi: Proteorhodopsin Phototrophy Promotes Survival of Marine Bacteria during Starvation. In: PLoS Biology . 8 (2010), p. E1000358. doi: 10.1371 / journal.pbio.1000358
- ↑ L. Gómez-Consarnau, JM González, M. Coll-Lladó, P. Gourdon, T. Pascher, R. Neutze, C. Pedrós-Alió, J. Pinhassi: Light stimulates growth of proteorhodopsin-containing marine Flavobacteria. In: Nature. 445, 2007, pp. 210-213 doi: 10.1038 / nature05381
- ↑ T. Riedel, J. Tomasch, I. Buchholz, J. Jacobs, M. Kollenberg, G. Gerdts, A. Wichels, T. Brinkhoff, H. Cypionka, I. Wagner-Döbler: Constitutive expression of the proteorhodopsin gene by a flavobacterium strain representative of the proteorhodopsin-producing microbial community in the North Sea. In: Applied and Environmental Microbiology. 76, 2010, pp. 3187-3197 doi: 10.1128 / AEM.02971-09
- ↑ J. Antón, A. Oren, S. Benlloch, F. Rodríguez-Valera, R. Amann, R. Rosselló-Mora: Salinibacter ruber gen. Nov., Sp. nov., a novel, extremely halophilic member of the Bacteria from saltern crystallizer ponds. In: International Journal of Systematic and Evolutionary Microbiology. 52, 2002, pp. 485-491. PMID 11931160 .
- ↑ JK Lanyi, SP Balashov: Xanthorhodopsin: a bacteriorhodopsin-like proton pump with a carotenoid antenna. In: Biochimica et Biophysica Acta (BBA). - Bioenergetics 1777, 2008, pp. 684-688. doi: 10.1016 / j.bbabio.2008.05.005
- ↑ MT Madigan, JM Martinko: Brock microbiology . Munich 2006, ISBN 3-8273-7187-2 , p. 375.
- ^ PJ Keeling: Diversity and evolutionary history of plastids and their hosts. In: American Journal of Botany. 2004, pp. 1481-1493 doi: 10.3732 / ajb.91.10.1481
- ↑ U. Sonnewald: Physiologie In: Strasburger textbook of botany. Heidelberg 2008, ISBN 978-3-8274-1455-7 , pp. 224-225.
- ↑ Kevin J. Flynn, Diane K. Stoecker, Aditee Mitra, John A. Raven, Patricia M. Glibert, Per Juel, Hansen Edna, Granéli, Joann M. Burkholder (2013): Misuse of the phytoplankton – zooplankton dichotomy: the need to assign organisms as mixotrophs within plankton functional types. Journal of Plankton Research 35 (1): 3-11. doi: 10.1093 / plankt / fbs062
- ↑ a b c Jarone Pinhassi, Edward F. DeLong, Oded Béjà, José M. González, Carlos Pedrós-Alió (2016): Marine Bacterial and Archaeal Ion-Pumping Rhodopsins: Genetic Diversity, Physiology, and Ecology. Microbiology and Molecular Biology Reviews 80 (4): 929-954. doi: 10.1128 / MMBR.00003-16
- ↑ DC Price, CX Chan, HS Yoon, EC Yang, H. Qiu, AP Weber, R. Schwacke, J. Gross, NA Blouin, C. Lane, A. Reyes-Prieto, DG Durnford, JA Neilson, BF Lang, G. Burger, JM Steiner, W. Löffelhardt, JE Meuser, MC Posewitz, S. Ball, MC Arias, B. Henrissat, PM Coutinho, SA Rensing, A. Symeonidi, H. Doddapaneni, BR Green, VD Rajah, J. Boore, D. Bhattacharya: Cyanophora paradoxa genome elucidates origin of photosynthesis in algae and plants. In: Science. 335, 2012, pp. 843-847 doi: 10.1126 / science.1213561
- ^ PJ Keeling: Diversity and evolutionary history of plastids and their hosts. In: American Journal of Botany. 2004, pp. 1483-1487. doi: 10.3732 / ajb.91.10.1481
- ↑ G. Hansen, L. Botes, MD Salas: Ultrastructure and large subunit rDNA sequences of Lepidodinium viride reveal a close relationship to Lepidodinium chlorophorum comb. nov. (= Gymnodinium chlorophorum). In: Phycological Research. 55, 2007, pp. 25–41 doi: 10.1111 / j.1440-1835.2006.00442.x (pdf)
- ↑ B. Imanian, Pombert JF, PJ Keeling: The Complete plastid genome of the Two 'Dino Toms' Durinskia baltica and Kryptoperidinium foliaceum. In: PLoS ONE. 5 (2010): e10711 doi: 10.1371 / journal.pone.0010711 (pdf)
- ↑ Žerdoner Čalasan A, Kretschmann J, Gottschling M. 2018. Absence of co-phylogeny indicates repeated diatom capture in dinophytes hosting a tertiary endosymbiont. Org Divers Evol 18: 29-38.
- ↑ JD Hackett, L. Maranda, HS Yoon, D. Bhattacharya: Phylogenetic evidence for the cryptophyte origin of the plastid of Dinophysis (Dinophysiales, Dinophyceae). In: Journal of Phycology. 39, 2003, pp. 440-448. doi: 10.1046 / j.1529-8817.2003.02100.x (pdf)
- ↑ K. Takishita, K. Koike, T. Maruyama, T. Ogata: Molecular evidence for plastid robbery (Kleptoplastidy) in Dinophysis, a dinoflagellate causing diarrhetic shellfish poisoning. In: Protist. 153, 2002, pp. 293-302 doi: 10.1078 / 1434-4610-00106
- ↑ K. Koike, H. Sekiguchi, A. Kobiyama, K. Takishita, M. Kawachi, K. Koike, T. Ogata: A Novel Type of Kleptoplastidy in Dinophysis (Dinophyceae): Presence of Haptophyte-type Plastid in Dinophysis mitra. In: Protist. 156, 2005, pp. 225-237 doi: 10.1016 / j.protis.2005.04.002
- ↑ T. Tengs, OJ Dahlberg, K. Shalchian-Tabrizi, D. Klaveness, K. Rudi, CF Delwiche, KS Jakobsen: Phylogenetic Analyzes Indicate that the 199Hexanoyloxy-fucoxanthin-Containing Dinoflagellates Have Tertiary Plastids of Haptophyte Origin. In: Molecular Biology and Evolution 17 (200) : 718-729. (pdf)
- ↑ ZB Yang, H. Takayama, K. Matsuoka, IJ Hodgkiss: Karenia digitata sp. nov. (Gymnodiniales, Dinophyceae), a new harmful algal bloom species from the coastal waters of west Japan and Hong Kong. In: Phycologia. 39, 2000, pp. 463-470. doi: 10.2216 / i0031-8884-39-6-463.1
- ↑ G. Hansen, N. Daugbjerg, P. Henriksen: Comparative study of Gymnodinium mikimotoi and Gymnodinium aureolum, comb. nov. (= Gyrodinium aureolum) based on morphology, pigment composition, and molecular data. In: Journal of Phycology. 36, 2000, pp. 394–410 doi: 10.1046 / j.1529-8817.2000.99172.x (pdf) ( Memento of the original from July 13, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ N. Daugbjerg, G. Hansen, L. Larsen, Ø. Moestrup: Phylogeny of some of the major genera of dinoflagellates based on infrastructure and partial LSU rDNA sequence data, including the erection of three new genera of unarmored dinoflagellates. In: Phycologia. 39, 2000, pp. 302-317. doi: 10.2216 / i0031-8884-39-4-302.1 (pdf)
- ^ W. Probst: Algae - omnipresent and versatile. In: teaching biology. 365, 2011, p. 3.
- ↑ B. Becker, B. Marin: Streptophyte algae and the origin of embryophytes. In: Annals of Botany. 103, 2008, pp. 999-1004.
- ↑ CH Wellman, PL Osterloff, U. Mohiuddin: Fragments of the earliest agricultural plants. In: Nature. 425, 2003, pp. 282-285.
- ^ U. Sonnewald: Physiology. In: Strasburger textbook of botany. Heidelberg 2008, ISBN 978-3-8274-1455-7 , pp. 224-225.
- ↑ Zhiling Guo, Huan Zhang, Senjie Lin (2014): Light-Promoted Rhodopsin Expression and Starvation Survival in the Marine Dinoflagellate Oxyrrhis marina. PLoS ONE 9 (12): e114941. doi: 10.1371 / journal.pone.0114941
- ^ PJ Keeling: Diversity and evolutionary history of plastids and their hosts. In: American Journal of Botany. 2004, p. 1487 doi: 10.3732 / ajb.91.10.1481
- ↑ DK Stoecker, MD Johnson, C. de Vargas, F. Not: Acquired phototrophy in aquatic protists - Supplement. In: Aquatic Microbial Ecology. 57, 2009, pp. 279-310 (pdf)
- ↑ C. Chantangsi, HJ Esson, BS Leander: Morphology and molecular phylogeny of a marine interstitial tetraflagellate with putative endosymbionts: Auranticordis quadriverberis n. Gen. Et sp. (Cercozoa). In: BMC Microbiology. 8, 2008, p. 123 doi: 10.1186 / 1471-2180-8-123
- ^ P. Hallock: Symbiote-bearing Foraminifera. In: BK Sen Gupta (ed.): Modern Foraminifera . Dordrecht 2002, ISBN 1-4020-0598-9 , pp. 123-140.
- ↑ N. Okamoto, I. Inouye: Hatena arenicola gen. Et sp. nov., a catablepharid undergoing probable plastid acquisition. In: Protist. 157, 2006, pp. 401-419 doi: 10.1016 / j.protis.2006.05.011
- ↑ CH Slamovits, N. Okamoto, L. Burri, ER James, PJ Keeling: A bacterial proteorhodopsin proton pump in marine eukaryotes. In: Nature Communications. 2, 2010, p. 183. doi: 10.1038 / ncomms1188
- ↑ MW Karakashian: Symbiosis in Paramecium Bursaria. In: Symposia of the Society of Experimental Biology. 29, 1975, pp. 145-173. PMID 785659 .
- ↑ HS Yoon, T. Nakayama, A. Reyes-Prieto, RA Andersen, SM Boo, K. Ishida, D. Bhattacharya: A single origin of the photosynthetic organelle in different Paulinella lineages. In: BMC Evolutionary Biology. 9, 2009, p. 98. doi: 10.1186 / 1471-2148-9-98
- ↑ T. Nakayama, Ki. Ishida: Another acquisition of a primary photosynthetic organelle is underway in Paulinella chromatophora. In: Current Biology. 19, 2009, pp. R284 – R285 doi: 10.1016 / j.cub.2009.02.043 (pdf)
- ↑ CP Queimaliños, BE Modenutti, EG Balseiro: Symbiotic association of the ciliate Ophrydium naumanni with Chlorella causing a deep chlorophyll a maximum in an oligotrophic South Andes lake. In: Journal of Plankton Research. 21, 1999, pp. 167–178 doi: 10.1093 / plankt / 21.1.167 (pdf)
- ^ G. Rambold, T. Friedl, A. Beck: Photobionts in lichens: Possible indicators of phylogenetic relationships? In: The Bryologist. 101, 1998, pp. 392-397.
- ↑ T. Pröschold, T. Darienko, PC Silva, W. Reisser, L. Krienitz: The systematics of zoochlorella revisited Employing at integrative approach. In: Environmental Microbiology. 13, 2011, pp. 350-364 doi: 10.1111 / j.1462-2920.2010.02333.x
- ↑ P. Brien: Contribution à létude de la régeneration naturelle chez les Spongillidae: Spongilla lacustris (L.) et Ephydatia fluviatilis (L.). In: Arch. Zool. exp. gene. 74, 1932, pp. 461-506.
- ↑ K. Dunn: Growth of endosymbiotic algae in the green hydra, Hydra viridissima. In: Journal of Cell Science. 88, 1987, pp. 571-578 (pdf)
- ↑ a b c A. Hauck: Two become one - algae as endosymbionts. In: teaching biology. 365, 2011, p. 37.
- ↑ a b D. Secord, L. Augustine: Biogeography and microhabitat variation in temperate algal-invertebrate symbioses: zooxanthellae and zoochlorella in two Pacific intertidal sea anemones, Anthopleura elegantissima and A. xanthogrammica. In: Invertebrate Biology. 119, 2000, pp. 139-146 doi: 10.1111 / j.1744-7410.2000.tb00002.x
- ^ AE Douglas: Growth and Reproduction of Convoluta Roscoffensis Containing Different Naturally Occurring Algal Symbionts. In: Journal of the Marine Biological Association of the United Kingdom. 65, 1985, pp. 871-879. doi: 10.1017 / S0025315400019378
- ^ PW Gilbert: The alga-egg relationship in Ambystoma maculatum, a case of symbiosis. In: Ecology. 25, 1944, pp. 366-369. doi: 10.2307 / 1931284
- ^ R. Kerney, E. Kim, RP Hangarter, AA Heiss, CD Bishop, BK Hall: Intracellular invasion of green algae in a salamander host. In: PNAS. 108, 2011, pp. 6497-6502 doi: 10.1073 / pnas.1018259108
- ↑ D. Riddle, "Types" of Zooxanthellae and What They Tell Us . pdf Appendix to: D. Riddle: Getting Really Up to Date on Zooxanthellae (Symbiodinium spp.). In: Advanced Aquarist. X (2011) link
- ↑ DL Taylor: In situ studies on the cytochemistry and infrastructure of a symbiotic marine dinoflagellate. In: Journal of the Marine Biological Association of the United Kingdom. 48, 1968, pp. 349-366 doi: 10.1017 / S0025315400034548
- ↑ S. K. Davy, I. Lucas, JR Turner: Uptake and Persistence of Homologous and Heterologous Zooxanthellae in the Temperate Sea Anemone Cereus pedunculatus (Pennant). In: The Biological Bulletin. 192, 1997, pp. 208-216. doi: 10.2307 / 1542715
- ^ DG Fautin, WK Fitt: A jellyfish-eating sea anemone (Cnidaria, Actiniaria) from Palau: Entacmaea medusivora sp. nov. In: Hydrobiologia. 216/217, 1991, pp. 453-461 doi: 10.1007 / BF00026499
- ^ JH Costello, PM Kremer: Circadian rhythmicity in the location of zooxanthellae of the scyphomedusa Linuche unguiculata. In: Marine Ecology Progress Series. 57, 1989, pp. 279–286 (pdf)
- ↑ JL Sachs, TP Wilcox: A shift to parasitism in the jellyfish symbiont Symbiodinium microadriaticum. In: Proceedings of the Royal Society B: Biological Sciences. 273, 2006, pp. 425-429 doi: 10.1098 / rspb.2005.3346
- ↑ O. Barneah, I. Brickner, M. Hooge, VM Weis, TC Lajeunesse: Three party symbiosis: acoelmorph worms, corals and unicellular algal symbionts in Eliat (Red Sea). In: Marine Biology. 151, 2007, pp. 1215-1223.
- ↑ O. Barneah, I. Brickner, M. Hooge, VM Weis, Y. Benayahu: First evidence of maternal transmission of algal endosymbionts at on oocyte stage in a triploblastic host, with observations on reproduction in Waminoa brickneri (Acoelomorpha). In: Invertebrate Biology. 126, 2007, pp. 113-119 doi: 10.1111 / j.1744-7410.2007.00082.x (free full text).
- ^ RK Trench, DS Wethey, JW Porter: Observations on the symbiosis with zooxanthellae among the Tridacnidae (Mollusca, Bivalvia). In: Biological Bulletin. 161, 1981, pp. 180-198 (pdf) .
- ^ R. Hinde, DC Smith: "Chloroplast symbiosis" and the extent to which it occurs in Sacoglossa (Gastropoda: Mollusca). In: Biological Journal of the Linnean Society. 6, 1974, pp. 349-356 doi: 10.1111 / j.1095-8312.1974.tb00729.x .
- ^ DL Taylor: Chloroplasts as symbiotic organelles in the digestive gland of Elysia viridis [Gastropoda: opisthobranchia]. In: Journal of the Marine Biological Association of the United Kingdom. 48, 1968, pp. 1-15.
- ↑ CV Mujer, DL Andrews, JR Manhart, SK Pierce, ME Rumpho: Chloroplast genes are expressed during the intracellular symbiotic association of Vaucheria litorea plastids with the sea slug Elysia chlorotica. In: PNAS. 93, 1996, pp. 12333-12338. (pdf)
- ↑ ME Rumpho, JM Worful, J. Lee, K. Kannan, MS Tyler, D. Bhattacharya, A. Moustafa, JR Manhart: Horizontal gene transfer of the algal nuclear gene psbO to the photosynthetic sea slug Elysia chlorotica. In: PNAS. 105, 2008, pp. 17867-17871. doi: 10.1073 / pnas.0804968105
- ↑ M. Plotkin, I. Hod, A. Zaban, SA Boden, DM Bagnall, D. Galushko, DJ Bergman: Solar energy harvesting in the epicuticle of the oriental hornet (Vespa orientalis). In: Natural Sciences. 97, 2010, pp. 1067-1076. doi: 10.1007 / s00114-010-0728-1 .