Günter Schultz
Günter Schultz (born January 23, 1936 in Frankfurt am Main ) is a German pharmacologist . He gained significant knowledge about biological signal processing . He has also introduced numerous younger people to this research area and thus shaped it, especially in Germany.
Life
His parents were the doctor Ernst-Gottfried Schultz and his wife Margarete geb. Eichner. After attending the Charlottenburg high school and graduating from high school in 1955, he studied medicine at the Free University of Berlin . In 1963 he received his license to practice medicine and was awarded a doctorate with a dissertation “Investigations into the morphological and secretory behavior of the corpus mucosa of the stomach in chronic hepatitis and liver cirrhosis ”. med. PhD . He joined Gerhard Senft's (1926–1967) working group at the Pharmacological Institute of the Free University, headed by Hans Herken . After Senft's death in 1968, he moved to the Pharmacological Institute of the Ruprecht-Karls-Universität Heidelberg , whose management Franz Gross took over in the same year . In 1970 he completed his habilitation in pharmacology in Heidelberg with a thesis entitled "Effects of hormones and pharmaceuticals on the metabolism of cyclic nucleoside-3'-5'-monophosphates in the rat kidney". In 1970 he also married the medical-technical assistant Karin Munske (1941–2009), a member of his group and co-author of some publications, first under her maiden name, then as Karin Schultz . From 1971 to 1973 he worked as a visiting scientist with Earl Wilbur Sutherland in the group of Joel Griffith Hardman (* 1933) at the Physiological Department of Vanderbilt University in Nashville , Tennessee . Sutherland discovered cyclic adenosine monophosphate (cAMP) as a second messenger in the action of chemical messengers such as adrenaline as well as the cAMP-forming enzyme adenylyl cyclase and the cAMP-cleaving enzyme phosphodiesterase , and for this he joined his laboratory in 1971, the year Schultz, received the Nobel Prize in Physiology or Medicine . From 1973 to 1983 Schultz held a C3 professorship in Heidelberg, but again spent a few months at Vanderbilt University. In 1983 he was appointed full professor at the Free University of Berlin and on October 1 he followed Hans Herken as director of the Pharmacological Institute on Thielallee in Dahlem . In 2003 he retired. The handover of the chair to his successor Walter Rosenthal , the head of the Berlin Research Institute for Molecular Pharmacology, later the Leibniz Institute for Molecular Pharmacology , occurred during the difficult time when the medical faculties of the Free University and the Humboldt University were merged .
research
Diuretics, cyclic AMP, and phosphodiesterase
Senft was interested in diuretics . Some, especially the thiazide diuretics and the chemically related but non-diuretic diazoxide , raised the blood sugar level . The Berlin group considered that cAMP could play a role, which regulates the carbohydrate metabolism . Schultz set up a method for measuring cAMP. Indeed, both diazoxide and the thiazide diuretics inhibited cAMP-degrading phosphodiesterase. It is believed that phosphodiesterase inhibition is not the main cause of the thiazide and diazoxide effects on blood sugar. With this work, however, Schultz had found his topic, biological signal processing.
Cyclic GMP and the guanylyl cyclase
Shortly before he left Berlin, a second cyclic nucleotide was discovered, cyclic guanosine monophosphate (cGMP). Schultz turned to him first in Heidelberg. “In the last few years the excretion of cyclic guanosine-3 ', 5'-monophosphate [..] in the urine of rats has been described [...]. So far, nothing is known about the formation and occurrence of this cyclic nucleotide in animal tissues. ”Schultz and his colleagues, including his first doctoral student Eycke Böhme (1943–1993), discovered“ formation and occurrence ”- and thus the cGMP-forming guanylyl cyclase , more precisely that Guanylyl cyclase dissolved in the cytosol - simultaneously with two US groups, including Hardman and Sutherland, in many organs. The guanylyl cyclase is the counterpart to the cAMP-forming adenylyl cyclase. It exists in several isoforms . The group in Heidelberg and later in Berlin continued to work on their purification and characterization through to structural elucidation by cloning their genes.
While searching for the function of cGMP, Schultz in Nashville found that noradrenaline , acetylcholine and carbachol , which cause the organ to contract, increased the cGMP content in the vas deferens of rats . However, cGMP was by no means a second messenger for the contraction - on the contrary. That became clear in 1977. As Schultz's group and the group of Ferid Murad found in the USA, several substances that cause the smooth muscles to relax, in particular the nitrovasodilators , also increase the level of cGMP. In addition, a cGMP derivative caused relaxation by itself. So cGMP was a second messenger not for contraction, but for relaxation. From 1980, with the recognition of nitric oxide as the body's own activator of cytosolic guanylyl cyclase - in other words, with the recognition of cytosolic guanylyl cyclase as the receptor for endogenous nitric oxide - the great physiological importance of the enzyme became clear.
Inhibition of adenylyl cyclase
Adrenaline triggers its effects through two types of receptors, the α and β adrenoceptors . Sutherland's stimulation of adenylyl cyclase occurs via β-adrenoceptors. As for the α-adrenoceptors, it was observed around 1970 that their activation reduced the cAMP content of organs. Schultz and his second Heidelberg doctoral student Karl-Heinrich Jakobs (* 1941) researched the mechanism on the membranes of thrombocytes (blood platelets). It consisted of adenylyl cyclase inhibition . So there was an opposing biological signal processing via the enzyme, stimulation and inhibition. Adrenaline inhibited platelet aggregation via β-adrenoceptors and stimulation of adenylyl cyclase, whereas it promoted platelet aggregation via α-adrenoceptors and inhibition of adenylyl cyclase. The α-adrenoceptors differed from those that induce smooth muscle contraction and were identified as α 2 -adrenoceptors in 1979 .
G proteins
G s , G i and beyond
It was also known since around 1970 that adrenaline and other messenger substances such as glucagon only stimulated adenylyl cyclase in the presence of guanosine triphosphate (GTP), whereby GTP was split into guanosine diphosphate (GDP) and phosphate ions. For this purpose, GTP did not come into contact with the receptor or adenylyl cyclase, but with a separate protein complex made up of three different subunits α, β and γ, a heterotrimeric G protein (GTP-binding protein). The inhibition of adenylyl cyclase in blood platelets by adrenaline was also found to be GTP-dependent. In addition to α 2 -adrenoceptors, adenylyl cyclase is inhibited by some muscarinic receptors , receptors for the neurotransmitter acetylcholine, for example in the heart. Again, the inhibition required the presence of GTP. The question of G-protein research around 1980 was "whether the same, or distinct, G-proteins confer activating and attenuating signals to the adenylate cyclase enzyme", whether it is one and the same G-protein or different G-proteins, which pass on the one hand stimulating and on the other hand inhibiting signals to the adenylyl cyclase.
Two Heidelberg experiments by Schultz, Jakobs and a post-doctoral student , Klaus Aktories , decided in 1983 for different G proteins. The first was carried out on lymphoma cells whose adenylyl cyclase no longer responded to stimulating messenger substances due to a genetic defect. The adenylyl cyclase-inhibiting messenger substance somatostatin, on the other hand, caused the usual inhibition in these cells: due to the genetic defect, the stimulating G protein G s - "s" for - "stimulating" was missing , but not the different inhibiting G protein G i - " i "for" inhibiting ". The second experiment was carried out on blood platelets whose adenylyl cyclase, as mentioned above, is inhibited by adrenaline via α 2 -adrenoceptors. The prostaglandin Prostaglandin-E 1 stimulates the platelet adenylyl cyclase. A bacterial toxin, the causative agent of whooping cough native pertussis toxin prevented the inhibition by adrenaline and the concomitant cleavage of GTP to GDP and phosphate ions, but not the stimulated by prostaglandin E 1 : pertussis toxin blocked G i , but not the different therefrom G s .
The G proteins have become one of the most intensively researched biological topics worldwide. In 1983 three were known, G s , G i and the transducin which serves to perceive light in the photoreceptor cells of the retina . In 1984 another was added which, like G i , was blocked by pertussis toxin, G o , in whose name “o” stands for “other”, “another” G protein. The variety has increased, especially through cloning of the genes. In 2005, 17 different genes for α, 5 different genes for β and 12 different genes for γ subunits were known, from which an even larger number of proteins resulted from alternative splicing . The heterotrimers composed of them are divided into four classes according to the α subunits, G s , G i / o , G q / 11 and G 12/13 . Via almost a thousand “G-protein-coupled receptors” , messenger substances, including many drugs , can act on the G-proteins and thus also on “effectors”, i.e. enzymes or ion channels .
G i , G o and the modulation of calcium channels
In 1987 and 1988 in Berlin, together with Walter Rosenthal and the Homburg electrophysiologists Wolfgang Trautwein and Jürgen Hescheler (* 1959), Schultz showed for the first time how voltage-controlled calcium channels were influenced by G proteins, initially in nerve cells. An opioid inhibited the entry of calcium ions into the cells. The inhibition was blocked by pertussis toxin. If a mixture of G i and G o was injected into the pertussis toxin-treated cells, the inhibition by the opioid reappeared: the inhibition was "reconstituted" by the injected G i -G o mixture . The publication immediately caused a sensation, especially since the responsible G protein was apparently G o , which for the first time could be assigned a role in signal processing.
The opposite role played G proteins in the adrenal cortex . In their cells, angiotensin II increased the entry of calcium through voltage-dependent calcium channels, and pertussis toxin blocked the increase. G o could be excluded immunologically and a G i protein identified. Finally, in a further endocrine gland , the anterior pituitary lobe - "GH 3 " cells from an anterior pituitary lobe tumor of rats - calcium entry was inhibited by somatostatin and increased by LHRH - the luteinizing hormone-releasing hormone . Pertussis toxin blocked both the inhibition and the increase. Inhibition and enhancement were apparently mediated by different G proteins, since the cells contained both G o and a G i protein.
Selectivity and complexity
From the immense number of G protein subunits and G protein heterotrimers, cells choose certain combinations that are appropriate for their respective functions. This first became clear through a "surprising series of experiments", "an astonishing series of experiments" on GH 3 anterior pituitary cells. Besides, as mentioned above, their calcium channels are also inhibited via somatostatin receptors via muscarinic receptors. Schultz, together with the molecular biologist Burghardt Wittig , their PhD student Christiane Kleuss and others, injected antisense oligonucleotides into the cell nuclei of GH 3 cells to precisely suppress the synthesis of certain G protein subunits, first α, then β and finally γ subunits and then treated the cells with somatostatin or carbachol. The conclusion in the third publication: “In each of the two signal translation pathways, a specific γ subunit appears to be complexed with a specific β subunit and a specific α o subunit, so that two specific G proteins result. … The muscarinic receptor is coupled to a G protein consisting of α o1 / β 3 / γ 4 , the somatostatin receptor to a G protein consisting of α o2 / β 1 / γ 3 . Both G-proteins mediate an inhibition of voltage-dependent calcium channels. "
Schultz's group used the same method to examine the signal processing at the GH 3 receptors for TRH - the thyrotropin- releasing hormone. Like LHRH (see above), TRH promoted calcium entry. The effect, more precisely the part of the effect that can be inhibited by pertussis toxin, was mediated by G proteins with the α subunits α i2 and α i3 .
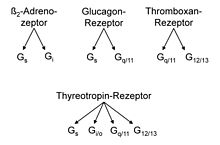
The doctoral student Karl-Ludwig Laugwitz (* 1968) and the post-doctoral student Stefan Offermanns developed another method for the analysis of G proteins, photoaffinity labeling followed by immunological identification. The signal processing at the thyroid receptors for the thyrotropin that stimulates the gland was examined. Effectors are adenylyl cyclase, which forms the second messenger cAMP, and a phospholipase C , which forms the second messenger inositol trisphosphate and diacylglycerol . Both enzymes are stimulated by thyrotropin. G-protein analysis initially showed that the G-protein classes G s and G q / 11 , and then that classes G i / o and G 12/13 were also involved, with a total of ten different α-subunits , an unexpected finding, as the authors write, which shows that, in addition to specific, multiple receptor G-protein couplings also occur.
On the other hand, signal cascades related to the effect of thromboxane A 2 and adenosine diphosphate on blood platelets were specific . Both are important mediators of platelet aggregation. Thromboxane A 2 activated G 12 and G 13 among other G proteins ; That was - in 1994 - the first evidence of a biological function of these two G proteins. Specifically via G 13 and the small GTPase Rho as an effector, thromboxane A 2 then triggered the change in shape of the platelets associated with the aggregation. Adenosine diphosphate, on the other hand, inhibited adenylyl cyclase via its receptor, the “purinoceptor” P2Y 12 , and G i2 .
G 12/13 and, as an effector, the small GTPase Rho were also involved in the contraction of the smooth muscles of blood vessels by endothelin -1 .
Selectivity and Complexity - “ Diversity and Selectivity ”: “The idea of a linear signal translation - a receptor couples to a G protein, which then activates an effector - does not explain the experimental findings. ... G-protein-mediated signal processing is rather a complex network with divergences and convergences at every step, from the receptor to the G-protein to the effector. "
TRP channels
Schultz 'most recent research area, from the early 1990s, goes back to mutagenesis studies on the fruit fly Drosophila melanogaster . One of numerous mutations had caused the photoreceptors to respond to exposure with too short an electric current, a transient receptor potential ; bright light therefore blinded the flies. From 1985 to 1989, the cause was recognized as the lack of a new calcium channel, a TRP channel , transient receptor potential channel , named after the malfunction in its deletion. Given the importance of calcium in biological signal processing, the search for similar ion channels in mammals began immediately. 1995 reported two US groups on the cloning of the Drosophila - trp gene homologous human gene. Schultz's group followed suit in 1996, but went further, expressing the homologous gene in animal cells ( CHO cells ) and indeed receiving a cation channel - the first mammalian TRP channel, TRPC1. It gained particular interest because the authors assumed that it could be the channel in the cell membrane through which cells replenish their calcium stores after they have been emptied, the CRAC channel , calcium release activated calcium channel , or store-operated channel .
After 1995-1996, TRP research proliferated rapidly. Schultz's group made a significant contribution to the cloning and characterization of several of the 26 TRP proteins known today (2013). In addition to TRP channels of the TRPC family, other proteins seem to contribute to the formation of the CRAC channels. Candidates as chemical signals for the opening of the CRAC channels are the second messengers inositol trisphosphate and diacylglycerol formed by phospholipase C. Indeed, diacylglycerol opened TRPC3 and TRPC6 channels. But also completely different stimuli, chemical and physical, open some TRP channels, such as cold and heat, capsaicin , which causes a feeling of warmth, and menthol , which causes a feeling of cold . The OTRPC4 or TRPV4 channel that Schultz's group cloned responded to changes in osmotic pressure in the extracellular space.
In 2000, Schultz and colleagues brought the TRP channels into an order with three families, which with extensions - six families - will exist.
Research organization
In addition to his work as a working group leader and institute director, Schultz devoted himself to higher-level tasks. In 1988 he founded the research focus of the German Research Foundation “Molecular Mechanisms of Signal Transduction in Membranes”, which he coordinated until 1994. In 1994 the research focus resulted in the Collaborative Research Center 366 (SFB 366) "Cellular Signal Recognition and Conversion", which existed until 2005, with the biochemist Werner Reutter as spokesman and Schultz as deputy spokesman. For a time he was part of eight sub-projects from the Pharmacological Institute. As part of the Dahlem Conferences , the SFB organized annual international conferences on “signal transduction”. From 1994 to 2000 Schultz was in the Senate of the German Research Foundation. He has long been the chief editor of the journal Molecular and Cellular Endocrinology and the Reviews of Physiology, Biochemistry and Pharmacology book series .
student
The following scientists completed their habilitation in Schultz's Heidelberg work group or his Berlin institute (year of habilitation):
- Franz Hofmann (1977), later professor for pharmacology and toxicology at the Technical University of Munich and founding director of the Berlin Research Institute for Molecular Pharmacology ; Hofmann's habilitation was co-supervised by Franz Gross
- Eycke Böhme (1978), later professor at the Berlin Institute; Franz Gross also supervised his habilitation
- Karl Heinrich Jakobs (1979), later professor for pharmacology and toxicology at the University of Essen
- Klaus Aktories (1983), later professor for pharmacology and toxicology at the Saarland University in Homburg and then at the Albert Ludwigs University of Freiburg
- Walter Rosenthal (1990), later appointed to Schultz's successor and since 2009 director of the Max Delbrück Center for Molecular Medicine in Berlin-Buch
- Roland Seifert (1992), later professor for pharmacology and toxicology at the University of Regensburg and then at the Hannover Medical School
- Doris Koesling (1996), later professor for pharmacology and toxicology at the Ruhr University Bochum
- Bernd Nürnberg (1997), later professor for biochemistry and molecular biology at Heinrich Heine University Düsseldorf and then professor for pharmacology and experimental therapy at Eberhard Karls University in Tübingen
- Thomas Gudermann (1998); later professor for pharmacology and toxicology at the Philipps University in Marburg and then at the Ludwig Maximilians University in Munich
- Stefan Offermanns (1998), later professor for pharmacology and toxicology at the Ruprecht-Karls-Universität Heidelberg and then director of the Max Planck Institute for Heart and Lung Research in Bad Nauheim
- Torsten Schöneberg (2001), later professor for molecular biochemistry at the University of Leipzig
- Michael Schaefer (2001), later professor for pharmacology and toxicology at the University of Leipzig
- Christian Harteneck (2003), later professor for pharmacology in Tübingen
recognition
In 1994 Schultz received the Max Planck Research Prize and in 1999 the Feldberg Prize for British-German scientific exchange . In 2001 he became a member of the German Academy of Sciences Leopoldina . In 2003 he was awarded the Federal Cross of Merit, 1st class .
literature
- Eberhard Hackenthal, Stefan Offermanns, Günter Schultz: Pharmacological Institute, Medical Faculty of the Ruprecht-Karls-Universität Heidelberg. In: Athineos Philippu (Ed.): History and work of the pharmacological, clinical-pharmacological and toxicological institutes in German-speaking countries, pp. 329–336. Berenkamp-Verlag, Innsbruck 2004. ISBN 3-85093-180-3 .
- Helmut Kewitz, Helmut Coper, Diether Neubert, Eckard Oberdisse, Konrad Keller, Günter Schultz: Institute for Pharmacology, Department of Human Medicine at the Free University of Berlin. In: Athineos Philippu (Ed.): History and work of the pharmacological, clinical-pharmacological and toxicological institutes in German-speaking countries, pp. 47–58. Berenkamp-Verlag, Innsbruck 2004. ISBN 3-85093-180-3 .
Individual evidence
- ↑ G. Senft, M. Hoffmann, K. Munske, G. Schultz: Effects of hydration and dehydration on cyclic adenosine 3 ′, 5′-monophosphate concentration in the rat kidney . In: Pflüger's archive for the entire physiology of humans and animals . 298, 1968, pp. 348-358. doi : 10.1007 / BF00363874 .
- ↑ G. Schultz, G. Senft, W. Losert, R. Sitt: Biochemical basis of diazoxide hyperglycemia . In: Naunyn-Schmiedeberg's archive for experimental pathology and pharmacology . 253, 1966, pp. 372-387. doi : 10.1007 / BF00245976 .
- ↑ G. Senft, W. Losert, G. Schultz, R. Sitt, HK Barthelheimer: Causes of the disorders in the carbohydrate metabolism under the influence of sulfonamidated diuretics . In: Naunyn-Schmiedeberg's archive for experimental pathology and pharmacology . 255, 1966, pp. 369-382. doi : 10.1007 / BF00593171 .
- ↑ a b E. Böhme, K. Munske, G. Schultz: Formation of cyclic guanosine-3 ', 5'-monophosphate in different tissues of the rat . In: Naunyn-Schmiedebergs Archive for Pharmacology . 264, 1969, pp. 220-221. doi : 10.1007 / BF02431417 .
- ↑ G. Schultz, E. Böhme, K. Munske: Guanyl cyclase. Determination of enzyme activity . In: Life Sciences . 8, No. 13, 1968, pp. 1323-1332. doi : 10.1016 / 0024-3205 (69) 90189-1 . PMID 5363364 .
- ↑ Doris Koesling, Eycke Böhme, Günter Schultz: Guanylyl cyclases, a growing family of signal-transducing enzymes . In: FASEB Journal . 5, No. 13, 1991, pp. 2785-2791. PMID 1680765 .
- ↑ Lincoln R. Potter: Guanylyl cyclase, structure and function . In: Cellular Signaling . 23, 2011, pp. 1921-1926. doi : 10.1016 / j.cellsig.2011.09.001 .
- ↑ Rupert Gerzer, Franz Hofmann, Günter Schultz: Purification of a soluble, sodium-nitroprusside-stimulated guanylate cyclase from bovine lung . In: European Journal of Biochemistry . 116, 1981, pp. 479-483. doi : 10.1111 / j.1432-1033.1981.tb05361.x . PMID 6114859 .
- ↑ Rupert Gerzer, Eycke Böhme, Franz Hofmann, Günter Schultz: Soluble guanylate cyclase purified from bovine lung contains heme and copper . In: FEBS Letters . 132, 1981, pp. 71-74. doi : 10.1016 / 0014-5793 (81) 80429-2 . PMID 6117479 .
- ↑ Doris Koesling, Joachim Herz, Heinrich Gausepohl, Feraydoon Niroomand, Klaus-Dieter Hinsch, Alexander Mülsch, Eycke Böhme, Günter Schultz, Rainer Frank: The primary structure of the 70 kDa subunit of bovine soluble guanylate cyclase . In: FEBS Letters . 239, No. 1, 1988, pp. 29-34. doi : 10.1016 / 0014-5793 (88) 80539-8 . PMID 2903071 .
- ↑ Christian Harteneck Barbara Wedei, Doris Koesling, Jürgen Malkewitz, Eycke Böhme, Gunter Schultz: Molecular cloning and expression of a new a-subunit of soluble guanylyl cyclase . In: FEBS Letters . 292, 1991, pp. 217-222. doi : 10.1016 / 0014-5793 (91) 80871-Y . PMID 1683630 .
- ↑ G. Schultz, JG Hardman, K. Schultz, JW Davis, EW Sutherland: A new enzymatic assay for guanosine 3 ′: 5′-cyclic monophosphate and its application to the ductus deferens of the rat . In: Proceedings of the National Academy of Sciences . 70, 1973, pp. 1721-1725. doi : 10.1073 / pnas.70.6.1721 . PMID 4352651 .
- ↑ G. Schultz, JG Hardman, K. Schultz, CE Baird, EW Sutherland: The importance of calcium ions for the regulation of guanosine 3 ': 5'-cyclic monophosphate levels . In: Proceedings of the National Academy of Sciences . 70, No. 12, 1973, pp. 3389-3393. doi : 10.1073 / pnas.70.12.3889 . PMID 4359494 .
- ↑ Klaus-Dieter Schultz, Karin Schultz, Günter Schultz: Sodium nitroprusside and other smooth muscle relaxants increase cyclic GMP levels in rat ductus deferens . In: Nature . 265, 1977, pp. 750-751. doi : 10.1038 / 265750a0 .
- ↑ Klaus-Dieter Schultz, Eycke Böhme, Volker AW Kreye, Günter Schultz: Relaxation of hormonally stimulated smooth muscular tissues by the 8-bromo derivative of cyclic GMP . In: Naunyn-Schmiedeberg's Archives of Pharmacology . 306, 1979, pp. 1-9. doi : 10.1007 / BF00515586 .
- ^ KH Jakobs, W. Saur, G. Schultz: Reduction of adenylate cyclase activity in lysates of human platelets by the alpha-adrenergic component of epinephrine . In: Journal of Cyclic Nucleotide Research . 2, 1976, pp. 381-392. PMID 14175 .
- ^ Karl H. Jakobs, Wilhelm Saur, Günter Schultz: Characterization of α- and β-adrenergic receptors linked to human platelet adenylate cyclase . In: Naunyn-Schmiedeberg's Archives of Pharmacology . 302, 1978, pp. 285-291. doi : 10.1007 / BF00508297 .
- ↑ Peter Lasch, Karl H. Jakobs: Agonistic and antagonistic effects of various α-adrenergic agonists in human platelets . In: Naunyn-Schmiedeberg's Archives of Pharmacology . 306, 1979, pp. 119-125. doi : 10.1007 / BF00498981 .
- ^ Karl H. Jakobs, Wilhelm Saur, Günter Schultz: Inhibition of platelet adenylate cyclase by epinephrine requires GTP . In: FEBS Letters . 85, 1978, pp. 167-170. doi : 10.1016 / 0014-5793 (78) 81272-1 . PMID 620788 .
- ^ Karl H. Jakobs, Klaus Aktories, Günter Schultz: GTP-dependent inhibition of cardiac adenylate cyclase by muscarinic cholinergic agonists . In: Naunyn-Schmiedeberg's Archives of Pharmacology . 310, 1979, pp. 113-119. doi : 10.1007 / BF00500275 .
- ↑ Lee E. Limbird: Activation and attenuation of adenylate cyclase. The role of GTP-binding proteins as macromolecular messengers in receptor - cyclase coupling . In: Biochemical Journal . 195, 1981, pp. 1-13. PMID 6272740 .
- ^ Karl H. Jakobs, Klaus Aktories, Günter Schultz: A nucleotide regulatory site for somatostatin inhibition of adenylate cyclase in S49 lymphoma cells . In: Nature . 303, 1983, pp. 177-178. doi : 10.1038 / 303177a0 . PMID 6140642 .
- ↑ Klaus Aktories, Günter Schultz, Karl H. Jakobs: Islet-activating protein impairs α 2 -adrenoceptor-mediated inhibitory regulation of human platelet adenylate cyclase . In: Naunyn-Schmiedeberg's Archives of Pharmacology . 324, 1983, pp. 196-200. doi : 10.1007 / BF00503894 .
- ↑ a b Nina Wettschureck, Stefan Offermanns: Mammalian G proteins and their cell type specific functions . In: Physiological Reviews . 85, 2005, pp. 1159-1204. doi : 10.1152 / physrev.00003.2005 .
- ↑ Franz Hofmann: Effects of pharmaceuticals on the organism: general pharmacodynamics. In: K. Aktories, U. Förstermann, F. Hofmann and K. Starke: General and special pharmacology and toxicology, pp. 4–24. 11th edition, Munich, Elsevier GmbH 2013. ISBN 978-3-437-42523-3
- ↑ J. Hescheler, W. Rosenthal, W. Trautwein, G. Schultz: The GTP-binding protein, G o , Regulates neuronal calcium channels . In: Nature . 325, 1987, pp. 445-447. doi : 10.1038 / 325445a0 .
- ↑ Kathleen Dunlap, George G. Holz, Stanley G. Rane: G proteins as regulators of ion channel function . In: Trends in Neurosciences . 10, 1987, pp. 241-244. doi : 10.1016 / 0166-2236 (87) 90166-4 .
- ↑ J. Hescheler, W. Rosenthal, KD Hinsch, M. Wulfern, W. Trautwein, G. Schultz: Angiotensin II-induced stimulation of voltage-dependent Ca 2+ currents in an adrenal cortical cell line. In: The EMBO journal. Volume 7, Number 3, March 1988, pp. 619-624, PMID 2456209 , PMC 454365 (free full text).
- ^ W. Rosenthal, J. Hescheler, KD Hinsch, K. Spicher, W. Trautwein, G. Schultz: Cyclic AMP-independent, dual regulation of voltage-dependent Ca 2+ currents by LHRH and somatostatin in a pituitary cell line. In: The EMBO journal. Volume 7, Number 6, June 1988, pp. 1627-1633, PMID 2458919 , PMC 457146 (free full text).
- ↑ David E. Clapham: Direct G protein activation of ion channels? . In: Annual Review of Neuroscience . 17, 1994, pp. 441-464. doi : 10.1146 / annurev.ne.17.030194.002301 .
- ↑ C. Kleuss, J. Hescheler, C. Ewel, W. Rosenthal, G. Schultz, B. Wittig: Assignment of G-protein subtypes to specific receptors inducing inhibition of calcium currents . In: Nature . 353, 1991, pp. 43-48. doi : 10.1038 / 353043a0 .
- ↑ C. Kleuss, H. Scherübl, J. Hescheler, G. Schultz, B. Wittig: Different β-subunits determine G-protein interaction with transmembrane receptors . In: Nature . 358, September, pp. 424-426. doi : 10.1038 / 358424a0 . PMID 1322501 .
- ↑ a b Christiane Kleuss, Hans Scherübl, Jürgen Hescheler, Günter Schultz, Burghardt Wittig: Selectivity in signal transduction determined by γ subunits of heterotrimeric G proteins . In: Science . 259, 1992, pp. 832-834. doi : 10.1126 / science.8094261 . PMID 8094261 .
- ↑ Maik Gollasch, Christiane Kleuss, Jürgen Hescheler, Burghardt Wittig, Günter Schultz: G i2 and protein kinase C are required for thyrotropin-releasinghormone-induced stimulation of voltage-dependent Ca 2+ channels in rat pituitary GH 3 cells . In: Proceedings of the National Academy of Sciences . 90, 1993, pp. 6265-6269. doi : 10.1073 / pnas.90.13.6265 . PMID 8392194 .
- ↑ Laugwitz completed his habilitation in Munich in 2002 and in 2006 became head of the I. Medical Clinic at the Klinikum rechts der Isar at the Technical University of Munich .
- ^ Anouk Allgeier, Stefan Offermanns, Jacqueline van Sande, Karsten Spicher, Günter Schultz, Jacques E. Dumont: The human thyrotropin receptor activates G-proteins G s and G q / 11 . In: Journal of Biological Chemistry . 269, 1994, pp. 13733-13735. PMID 8188646 .
- ^ Karl-Ludwig Laugwitz, Anouk Allgeier, Stefan Offermanns, Karsten Spicher, Jacqueline van Sande, Jaques E. Dumond, Günter Schultz: The human thyrotropin receptor: A heptahelical receptor capable of stimulating members of all four G protein families . In: Proceedings of the National Academy of Sciences . 93, 1996, pp. 116-120. doi : 10.1073 / pnas.93.1.116 . PMID 8552586 .
- ^ Stefan Offermanns, Karl-Ludwig Laugwitz, Karsten Spicher, Günter Schultz: G proteins of the G 12 family are activated via thromboxane A 2 and thrombin receptors in human platelets . In: Proceedings of the National Academy of Sciences . 91, 1994, pp. 504-508. doi : 10.1073 / pnas.91.2.504 . PMID 8290554 .
- ↑ Birgit Klages, Ursula Brandt, Melvin I. Simon, Günter Schultz, Stefan Offermanns: Activation of G 12 / G 13 results in shape change and Rho / Rho-kinase-mediated myosin light chain phosphorylation in mouse platelets . In: Journal of Cell Biology . 144, 1999, pp. 745-754. doi : 10.1083 / jcb.144.4.745 . PMID 10037795 .
- ^
- ^ Antje Gohla, Günter Schultz, Stefan Offermanns: Role for G 12 / G 13 in agonist-induced vascular smooth muscle cell contraction . In: Circulation Research . 2000. doi : 10.1161 / 01.RES.87.3.221 . PMID 10926873 .
- ↑ Thomas Gudermann, Frank Kalkbrenner, Günter Schultz: Diversity and Selectivity of receptor-G protein interaction . In: Annual Review of Pharmacology and Toxicology . 36, 1996, pp. 429-459. doi : 10.1146 / annurev.pa.36.040196.002241 .
- ↑ Craig Montell: The history of TRP channels, a commentary and reflection . In: Pflüger's archive . 461, 2011, pp. 400-506. doi : 10.1007 / s00424-010-0920-3 . PMID 21287198 .
- ↑ Christof Zitt, Andrea Zobel, Alexander G. Obukhov, Christian Harteneck, Frank Kalkbrenner, Andreas Lückhoff, Günter Schultz: Cloning and functional expression of a human Ca 2+ -permeable cation channel activated by calcium store depletion . In: Neuron . 16, 1996, pp. 1189-196. doi : 10.1016 / S0896-6273 (00) 80145-2 . PMID 8663995 .
- ↑ a b Stephen PH Alexander, Alistair Mathie, John A. Peters: Transient receptor potential (TRP) cation channels . In: British Journal of Pharmacology . 164, supplement 1, 2011, pp. S166-S174. doi : 10.1111 / j.1476-5381.2011.01649_1.x .
- ↑ " Orai " and " STIM ". On this: Lutz Birnbaumer: The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca 2+ concentrations . In: Annual Review of Pharmacology and Toxicology . 49, 2009, pp. 395-426. doi : 10.1146 / annurev.pharmtox.48.113006.094928 .
- ↑ Thomas Hofmann, Alexander G. Obukhov, Michael Schaefer, Christian Harteneck, Thomas Gudermann, Günter Schultz: Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol . In: Nature . 397, 1999, pp. 259-263. doi : 10.1038 / 16711 . PMID 9930701 .
- ^ Rainer Strotmann, Christian Harteneck, Karin Nunnenmacher, Günter Schultz, Tim D. Plant: OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity . In: Nature New Biology . 2, 2000, pp. 695-702. doi : 10.1038 / 35036318 . PMID 11025659 .
- ↑ Christian Harteneck, Tim D. Plant, Günter Schultz: From worm to man: three subfamilies of TRP channels . In: Trends in Neurosciences . 23, 2000, pp. 159-166. doi : 10.1016 / S0166-2236 (99) 01532-5 . PMID 10717675 .
- ^ David E. Clapham, David Julius, Craig Montell, Günter Schultz: International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels . In: Pharmacological Reviews . 57, 2005, pp. 427-450. doi : 10.1124 / pr.57.4.6 .
- ^ Member entry by Günter Schultz (with picture) at the German Academy of Natural Scientists Leopoldina , accessed on July 22, 2016.
| personal data | |
|---|---|
| SURNAME | Schultz, Günter |
| BRIEF DESCRIPTION | German physician and pharmacologist |
| DATE OF BIRTH | January 23, 1936 |
| PLACE OF BIRTH | Frankfurt am Main |