Camphor
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
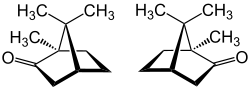
|
||||||||||||||||||||||
| (+) - camphor (left) and (-) - camphor (right) | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Camphor | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 10 H 16 O | |||||||||||||||||||||
| Brief description |
colorless solid with an aromatic odor |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 152.23 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1 g cm −3 [(±) camphor] |
|||||||||||||||||||||
| Melting point |
179 ° C [(±) camphor] |
|||||||||||||||||||||
| boiling point |
209 ° C [(±) camphor] |
|||||||||||||||||||||
| solubility |
soluble in water (1.5 g l −1 at 20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
|
|||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Camphor or Camphora (technical language, standard language: camphor ) is a colorless solid obtained from the wood of camphor trees by steam distillation . He is a bicyclic monoterpene - ketone and is formally derived from Bornan from. There are two enantiomers of camphor, (+) - camphor [synonym: (1 R , 4 R ) camphor] and (-) - camphor [synonym: (1 S , 4 S ) camphor]. The structure was clarified by Julius Bredt .
Camphor occurs naturally in various plants and is produced synthetically on an industrial scale. It is medicinally effective, psychoactive and toxic in improper doses and classified as a less dangerous substance and is used, among other things, as a component of various technical and medical products.
Camphor is distilled from the wood, stumps , twigs and leaves of a camphor tree, until the 16th century from the Borneo camphor tree ( Dryobalanops aromatica Gaertn., Synonym Dryobalanops camphora ), from the 16th century mainly from the camphor tree ( Cinnamomum camphora ). Known since late antiquity (5th century) under the Greek name kaphoura (καφουρά), which goes back to Sanskrit karpura ('white') or Prakrit kappura . The Indian name, in turn, was borrowed from an Austronesian language in Sumatra . Middle High German and Early New High German and until the 19th century camphor or the Harz from the camphor tree was also called Gaffer , regionally also Gauffer .
Occurrence
Both enantiomers of camphor [(+) - camphor or (-) - camphor] and the racemate (±) camphor occur in nature. Camphor is mainly found in the essential oils of the bay family , daisy family, and mint family . (+) - Camphor occurs in the bark and resin of the camphor tree ( Cinnamomum camphora ), an evergreen tree that grows mainly in Asia .
properties
Camphor is a colorless or white, mostly crumbly and chunky tough powder made of waxy crystals . With ethanol can rhombohedral crystals are generated. When quenching molten camphor, cubic crystals form. Camphor has a characteristic, strong, fragrant, aromatic-woody, eucalyptus- like odor. The taste is pungent and bitter, also slightly cooling like menthol . It melts at 179 ° C and boils at 209 ° C. The powder is hardly soluble in water (1.5 g per liter of water); in contrast, it dissolves well in ethanol. In addition, it is very easily soluble in petroleum ether , easily soluble in ether, acetone, chloroform and in fatty oils and very sparingly soluble in glycerine . It forms colorless solutions with ethanol, from which the camphor separates out again when water is added. The density is 0.96 g / cm 3 . Camphor is highly volatile and sublimes even at room temperature. It burns with a sooty flame. With camphor, the molar lowering of the melting point is noticeably large, it is 39.7 K · (kg / mol ). Camphor liquefies when it comes into contact with menthol, naphthol or chloral hydrate . The specific angle of rotation is + 48 °.
The reduction of (+) - camphor [(1 R , 4 R ) -camphor] with sodium borohydride or lithium aluminum hydride produces 95% isoborneol [(1 R , 2 R , 4 R ) -isoborneol] and 5% of its stereoisomer borneol [(1 R , 2 S , 4 R ) -Borneol]:
Camphor is slightly hazardous to water ( WGK 1).
Safety-related parameters
Camphor forms flammable vapor-air mixtures at high temperatures. The compound has a flash point of 77 ° C. The explosion range is between 0.6% by volume (38 g / m 3 ) as the lower explosion limit (LEL) and 4.5% by volume (280 g / m 3 ) as the upper explosion limit (UEL). The ignition temperature is 460 ° C. The substance therefore falls into temperature class T1.
Effects and side effects
Camphor acts on the central nervous system and the kidneys , in higher doses also ( analeptically ) on the respiratory center . It stimulates the blood circulation and dissolves mucus, but can also lead to nausea, fear, shortness of breath and agitation. Taken orally in overdose, it leads to states of confusion and twilight, depersonalization , extreme déjà vu experiences , panic and acute profound disorders of the short-term memory up to amnesia and epileptic seizures . The lethal dose for an adult is 0.1 g / kg body mass. The metabolism initially goes to the camphor-derived alcohol 2- borneol , which is further converted to glucuronide in the liver . This is finally excreted in the urine.
use
Chemical products
Camphor is used in fireworks , partly in explosive gelatine and in moth repellants. It is also used for celluloid production and as a plasticizer for cellulose esters .
Biological and physical applications
In bee care, camphor is used as an EU-approved active ingredient against mite infestation and is often also used in entomological collections for disinfection and for protection against collection pests. It is also used in storm glass barometers .
Because of its high cryoscopic constant of 39.7 K · (kg / mol), camphor was previously used to determine the molar mass according to Beckmann .
Medicine and cosmetics

Camphor is added to cosmetic and medical preparations, for example in products for muscle strains, rheumatism or neuralgia , in aftershaves such as Pitralon , in remedies for colds (for example, Wick VapoRub contains 5% camphor), in dentistry for disinfecting infected root canals. In the past it was also used as an analeptic , now less often due to its effects on the heart and circulation.
In the Middle Ages, camphor was stretched by adding " varnish ".
Luxury and intoxicants
Rare cases of the use of camphor as an intoxicant are known. The effects of inhaling camphor show up in fits of laughter despite pain in the airways. Camphor is still used in snuff from England, he whereas in Germany, according to tobacco regulation to the prohibited substances in tobacco is one and can not be added. Historically in China it was used as a flavor and structure enhancing additive in ice cream .
Religious practice
Alone or in connection with tree resins and / or other substances, it is used as an incense when smoking . Furthermore, in Islam the deceased are often cleaned during the last ablution with washing water to which camphor has been added for perfuming.
Pharmacological properties and uses
Camphor is used in a 2.5% solution for hypotonic or orthostatic dysregulations for peroral or oromucosal administration (via the oral mucosa). In addition, it is used externally in the form of 10% ointments, higher concentrations can lead to severe poisoning. The ointments stimulate blood circulation and are used for chronic arthritis , tendinitis , traumatic swelling , myalgia , bursitis , strains , sprains and inflammatory edema . Concentrations of 0.1% have a low local anesthetic effect and a cooling effect due to the irritation of nerve endings that conduct cold sensations. Camphor is quickly absorbed through the skin and mainly reaches the fatty tissue via the blood plasma. It crosses the blood-brain barrier , the blood-milk barrier and the placental barrier . The metabolism takes place to carboxylic acids and / or camphor alcohols, the active ingredient is partially glucuronidated . Excretion takes place primarily via the kidneys, and to a lesser extent via the lungs, faeces and milk.
Camphor is the oldest analeptic . In a suitable dose range, analeptics increase the activity of certain sections of the central nervous system. The pharmacological use of camphor is now considered obsolete. The earlier common use of camphor together with pentetrazole (brand name Cardiazol) in cardiazole shock therapy as the treatment of spasms for mental illnesses reached a peak around 1938.
Extraction and presentation
Camphor can be produced synthetically, but it can also be obtained by steam distillation and crystallization from shredded plant parts of the camphor tree . Natural camphor is mostly right-handed [(+) - camphor, "Japanese camphor"]. In Matricaria species there is also left-turning (-) camphor ("Matricaria camphor"). Nowadays, camphor is technically synthesized starting from α- pinene . The intermediate carbonium ion mainly produces the thermodynamically more stable isobornyl acetate , which is then oxidized to (-) - camphor after hydrolysis of the ester .
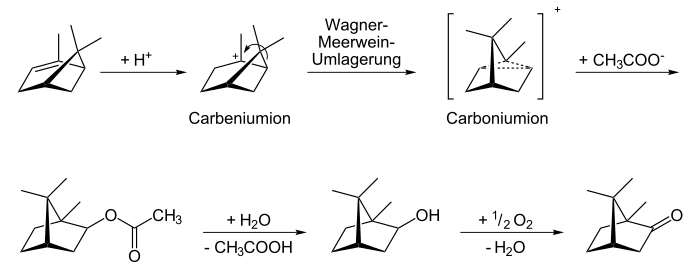
α- pinene is protonated; by Wagner-Meerwein rearrangement it is rearranged to Bornan in the form of a carbonium ion. This reacts with sodium acetate to form isobornyl acetate , which is then hydrolyzed to isoborneol and finally oxidized to camphor.
The total synthesis of camphor succeeded Gustaf Komppa in 1903 (racemate) and 1905. Already before (1902) camphor was synthesized industrially from turpentine oils (pinenes) at Schering in Berlin under the direction of Ossian Aschan .
biosynthesis
From geranyl pyrophosphate via cyclization of linalool pyrophosphate to bornyl pyrophosphate followed by hydrolysis to borneol and subsequent oxidation, camphor is enzymatically formed in sage ( Salvia officinalis ).
Web links
- Entry to camphor . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD
Individual evidence
- ↑ a b c d e f g h i j k l m Entry on camphor in the GESTIS substance database of the IFA , accessed on February 23, 2016(JavaScript required) .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 76-22-2 or camphor ), accessed on November 2, 2015.
- ↑ a b entry on camphor. In: Römpp Online . Georg Thieme Verlag, accessed on June 12, 2014.
- ↑ Camphor data sheet at Sigma-Aldrich , accessed on May 8, 2017 ( PDF ).
- ^ Willem Hendrik de Vriese : De Kampferboom van Sumatra, Dryobalanops Camphora Colebr., Volgens Dr. F. Junghuhn's Waarnemingen op de Plaats zelve, en door nadere Onderzoekingen toegelicht. Den Hoogleeraar CGC Reinwardt, bij de Herinnering to zijn vijftigjarig Hoogleeraar-Ambt, on the 10th of June 1851, Aangebden. Leiden 1851 ( online ).
- ↑ Otto Beßler: The identification of medieval drugs and medicinal plants. Stuttgart 1958 (= publications of the International Society for the History of Pharmacy, New Series, 13), p. 47 f.
- ↑ Early New High German Dictionary: gaffer. .
- ↑ Baierische Pharmacopoe (1823)
- ↑ Jürgen Martin: The 'Ulmer Wundarznei'. Introduction - Text - Glossary on a monument to German specialist prose from the 15th century. Königshausen & Neumann, Würzburg 1991 (= Würzburg medical-historical research. Volume 52), ISBN 3-88479-801-4 (also medical dissertation Würzburg 1990), pp. 130 and 141.
- ^ A b The Merck Index : An Encyclopedia of Chemicals, Drugs, and Biologicals. 14th Edition (Merck & Co., Inc.), Whitehouse Station, NJ, USA 2006, ISBN 978-0-911910-00-1 , p. 279.
- ↑ Jonathan Clayden, Nick Greeves, Stuart Warren, Peter Wothers: Organic Chemistry. Oxford University Press, 2001, ISBN 978-0-19-850346-0 , p. 862.
- ^ E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases , Wirtschaftsverlag NW - Verlag für neue Wissenschaft GmbH, Bremerhaven 2003.
- ↑ a b Wolfgang Löscher et al .: Pharmacotherapy in house and farm animals. 7th ed., Paul Parey, 2006, pp. 503-504.
- ↑ Auterhoff -Knabe-Höltje: Textbook of pharmaceutical chemistry. Wissenschaftliche Verlagsgesellschaft Stuttgart 1999, ISBN 3-8047-1645-8 .
- ↑ WICK VapoRub ointment , composition, accessed March 15, 2015.
- ↑ Konrad Goehl : Observations and additions to the 'Circa instans'. In: Medical historical messages. Journal for the history of science and specialist prose research. Volume 34, 2015 (2016), pp. 69-77, here: p. 70.
- ^ A Sweet History of an Icy Treat , in: History Cooperative, December 15, 2015.
- ^ Anne-Sophie Fröhlich: Priestly tasks in Sunni Islam. LIT Verlag, Berlin-Hamburg-Münster 1997, ISBN 3-8258-3045-4 , p. 44.
- ^ Paul Walden, History of Organic Chemistry since 1880, Springer, 1990, p. 557.
- ↑ Rodney Croteau, Frank Karp: Biosynthesis of monoterpenes: Hydrolysis of bornyl pyrophosphate, an essential step in camphor biosynthesis, and hydrolysis of geranyl pyrophosphate, the acyclic precursor of camphor, by enzymes from sage (Salvia officinalis) . In: Archives of Biochemistry and Biophysics . 198, No. 2, 1979, pp. 523-532. doi : 10.1016 / 0003-9861 (79) 90527-7 .




