Magaldrat
| General | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-proprietary name | Magaldrat | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | Al 5 Mg 10 (OH) 31 (SO 4 ) 2 x H 2 O | ||||||||||||||||||
| Brief description |
white, crystalline and odorless powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| Mechanism of action | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 1097.30 g · mol -1 (anhydrous) | ||||||||||||||||||
| solubility |
practically insoluble in water and ethanol 96%, easily soluble in dilute inorganic acids |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Magaldrate is a drug from the group of layer lattice antacids , which have a molecular layer lattice structure and are used for the symptomatic therapy of diseases in which excess gastric acid is to be bound.
Clinical information
application areas
Magaldrat is suitable for the symptomatic treatment of hyperacidity and its acute consequences: dyspeptic complaints in the upper abdomen , reflux esophagitis , gastritis (inflammation of the stomach lining ), bloating and acid-related stomach problems. Magaldrate can be used for rapid symptomatic therapy - to proton pump inhibitors act - during peptic ulcer ( gastric ulcer ) or duodenal ulcer ( duodenal ulcer ) are used.
Contraindications
In the case of known hypersensitivity to Magaldrat, the agent must not be taken.
Drug interactions
They are pharmacokinetic interactions: antacids can affect the absorption, distribution, or excretion of concomitantly administered drugs. Therefore, there should always be an interval of 2 hours between taking magaldrat and other drugs. Among the drugs which interactions cause with magaldrate include, for example digoxin , benzodiazepines , bisphosphonates , coumarin derivatives , indomethacin , cimetidine , azole - antifungal agents , chenodeoxycholic acid and ursodeoxycholic acid . In particular, a considerable reduction in the absorption of tetracyclines and quinolone derivatives ( ciprofloxacin , ofloxacin , norfloxacin ) was observed when taking antacids , so that taking magaldrat cannot be recommended during therapy with these antibiotics. The simultaneous intake of aluminum-containing antacids with acidic drinks (e.g. fruit juices , wine ) increases the intestinal absorption of aluminum. This property applies to effervescent tablets that contain citric acid or tartaric acid . Magaldrate also impairs iron absorption due to the increased pH value of gastric juice .
Use during pregnancy and breastfeeding
For use in pregnancy neither controlled trials are currently in pregnant women in animals. Studies in animals have for aluminum - salts a reproductive toxicity shown. However, studies have shown that the aluminum levels are clearly increased after taking aluminum salts, while the levels after taking magaldrate, because of the layered lattice structure , do not differ from the values without antacid administration. Nevertheless, the preparation should only be used during pregnancy if strictly indicated , for short periods and in the lowest possible dose. Aluminum compounds pass into breast milk . A risk for the newborn is not to be assumed since only small amounts are absorbed.
Special patient groups
(Magaldrat in patients with impaired renal function should creatinine - clearance <30 ml / min) only for regular monitoring of the aluminum and magnesium plasma levels are administered. The serum aluminum level should not exceed 40 µg · L −1 . Even with long-term use, regular medical checks and the determination of the aluminum and magnesium plasma levels are necessary. The drug should not be used to treat children under 12 years of age as there is insufficient experience in this age group.
unwanted effects
Soft stools are very common and diarrhea is very rare . With long-term use in high doses, systemic toxicity is possible due to the increased aluminum and magnesium levels . Aluminum can accumulate in the bones and central nervous system (CNS) with subsequent encephalopathy . The reduction in phosphate - plasma levels ( hypophosphatemia ), a very rare osteomalacia (painful osteomalacia trigger).
Pharmacological properties
Physiological basis of gastric acid production
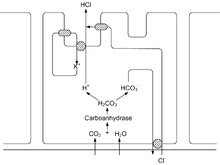
The various secretory cells of the gastric mucosa - secondary cells , parietal cells and main cells - secrete 1–3 liters of gastric juice daily . The gastric juice is a colorless, slightly opalescent , aqueous liquid with a pH of 0.9–1.5. The secretion is stimulated and regulated by mechanical and chemical irritation of the stomach wall, by local hormones of the digestive tract ( gastrin , secretin , histamine ) and by the vagus nerve . The parietal cells, ( Latin Exocrinocyti parietales, also called parietal cells ), allow the secretion of hydrogen (+ I) ions H + and chlorine (−I) ions Cl - , which is a blood isotonic hydrochloric acid (150 mmol L −1 , pH 0.9). The hydrochloric acid not only converts the gastric digestive enzyme, pepsin , into its effective form, but also renders a large part of the bacteria ingested with food harmless. The protons come from the carbonic acid-hydrogen carbonate equilibrium,
- ( Carbonic anhydrase reaction)
which is catalyzed by the carbonic anhydrase of the parietal cells and shifted to the side of proton formation. The protons are pumped into the lumen-sided canaliculi with the help of the proton pump H + / K + -ATPase in exchange for K + -ions . Following the concentration gradient, K + then diffuses back into the canaliculus via a potassium channel , where it is again available for exchange with H + . Bicarbonate is released into the blood in exchange for Cl - . Cl - then enters the canaliculus following the electrical gradient via a special chloride channel . From the canaliculus, H + and Cl - enter the lumen , while K + is taken back into the cell. This creates a proton gradient with an intracellular to luminal H + concentration ratio of 1:10 6 . The energy for maintaining this high gradient comes from the hydrolysis of a molecule of adenosine 5′-triphosphate (ATP) to adenosine 5′-diphosphate (ADP) for the transport of 2 H + in each case . Moreover secrete the parietal cells for the vitamin B 12 reabsorption in the ileum necessary intrinsic factor , an about 50 kDa heavy glycoprotein with a high content of N -Acetylneuraminsäure , which prior to cleavage by proteases protects.
Mechanism of action
Magaldrate is a stable complex compound with a defined crystalline layer-lattice structure, a so-called Schichtgitterantazidum ( engl. : Lattice structure antacid ), in which magnesium and aluminum bound structurally fixed in superposed mesh layers. The antacid effect is by binding protons (H + ) by the sulfate - anions [SO 4 ] 2- or hydroxide ions OH - the interstitial layer about. With the neutralization the lattice structure dissolves and above a pH value of 5 the reaction comes to a standstill. This acid buffering results in a constant pH range of 3–5 in the gastric juice. 1 g magaldrate neutralizes about 27 meq hydrochloric acid .
In addition to the buffering effect, there is a dose- and pH-dependent chemical binding of cytotoxic bile acids and lysolecithin that have entered the stomach through duodenogastric reflux . These aggressors - along with H. pylori - are held responsible for the development of gastritis and gastric ulcers . It also forms a layer that protects the stomach lining from damage. The peristalsis of the stomach and intestines are not affected by magaldrate, and it does not cause acid - rebound .
Absorption and distribution in the body
Magaldrat is practically not absorbed from the gastrointestinal tract . During the neutralization process, a small amount of magnesium and aluminum ions are released. A very small proportion of the aluminum cations and a maximum of 5% of the magnesium ions are absorbed depending on the pH.
Metabolism : Unabsorbed cations are mainly converted into poorly soluble phosphates during the intestinal passage and excreted in the stool . Resorbed Al 3+ ions are bound to plasma proteins . Especially with impaired kidney function and / or long intake and high dosage, deposits in bones, CNS and other organs can occur after the plasma protein binding capacity is exceeded . The concentration of magnesium and aluminum in the blood serum remains unchanged; however, slightly elevated serum levels of aluminum have occasionally been found in patients with healthy kidneys.
The elimination of magaldrate is via the digestive tract and the small amount of the absorbed aluminum ions is renally excreted .
Chemical and pharmaceutical information
Magaldrate is the international nonproprietary name for the Schichtgitterantazidum consisting of aluminum and magnesium hydroxides , and Al 3+ and Mg 2+ - sulfates . The composition of the active ingredient corresponds roughly to the formula Al 5 Mg 10 (OH) 31 (SO 4 ) 2 • x H 2 O and according to Ph. Eur. 5.0-5.8 2006 contains at least 90.0% and at most 105.0% magaldrate, calculated on the dried substance. It loses 10–20% of its weight when dried for 4 hours at 200 ° C in a drying cabinet .
Development and marketing
Magaldrat was first synthesized by the German chemist Gunther Hallmann and published as a patent on February 2, 1960 by the then Byk Gulden Lomberg, Chemische Fabrik GmbH in Konstanz . Byk Gulden received approval for Riopan ( original preparation ) in Germany in 1983. The company was renamed Altana Pharma in 2002 , the pharmaceutical division of which was sold to Nycomed in 2007 , which in turn was taken over in 2011 by the international pharmaceutical company Takeda . In 2013, Dr. Kade approved Riopan (gastric gel and chewable tablets) for Germany from Takeda.
Dosage forms and trade names
Magaldrate-containing finished preparations are available as tablets, chewable tablets, lozenges, suspensions and stomach gels with an active ingredient content of 400 mg – 1600 mg per single dose . In Germany and Austria all preparations are compulsory for pharmacies . In Switzerland, the drugs are classified in dispensing category D and can be obtained without a prescription in pharmacies and drugstores after receiving medical advice .
Trade names: Bisco-Magaldrat (D), Gastripan (D), Glysan (D), Magastron (S), Marax (D), Riopan (D, A, CH), Magaldrat-ratiopharm chewable tablets (D) and others
Web links
- Patent EP0154114 : Method for preparing magaldrate. Registered on January 12, 1985 , published on September 11, 1985 , applicant: Giulini Chemie , inventor: Klaus Schanz.
literature
- European Pharmacopoeia Commission (Ed.): EUROPEAN PHARMACOPOE 5TH EDITION . tape 5.0-5.8 , 2006.
- Ernst Mutschler et al .: Mutschler - drug effects textbook of pharmacology and toxicology . 9th edition. Scientific Verlagsgesellschaft, Stuttgart 2008, ISBN 978-3-8047-1952-1 .
- Franz v. Bruchhausen (Editor), Siegfried Ebel (Editor), Eberhard Hackenthal (Editor), Ulrike Holzgrabe (Editor): Hager's Handbuch der Pharmazeutischen Praxis: sequence Volume 5: L-Z substances . Springer, ISBN 3-540-62646-8 .
- Hermann J. Roth: Medicinal Chemistry: Targets and Drugs; 157 tables . German Apotheker-Verlag, Stuttgart 2005, ISBN 3-7692-3483-9 .
- Dieter Strauss: Chemistry for pharmaceutical practice: textbook and reference work . German Apotheker-Verlag, Stuttgart 2000, ISBN 3-7692-2606-2 .
- Bert Ehgartner: Dirty little secret - the aluminum files , Verlag Ennsthaler 2012, ISBN 3-85068-894-1 .
Individual evidence
- ↑ a b c European Pharmacopoeia Commission (Ed.): EUROPÄISCHE PHARMACOPÖE 5th EDITION . tape 5.0-5.8 , 2006.
- ↑ There is not yet a harmonized classification for this substance . A labeling of Aluminum magnesium hydroxide sulfate (Al5Mg10 (OH) 31 (SO4) 2), hydrate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on July 7, 2020, is reproduced from a self-classification by the distributor .
- ↑ a b c d e f Sample text of the specialist information for Magaldrat of the Federal Institute for Drugs and Medical Devices (BfArM), as of October 2003.
- ↑ Pharmavista interaction database .
- ↑ W. Forth, D. Henschler, W. Rummel: General and special pharmacology and toxicology . 9th edition. URBAN & FISCHER, Munich 2005, ISBN 3-437-42521-8 .
- ↑ Entry on gastric juice. In: Römpp Online . Georg Thieme Verlag, accessed on June 5, 2014.
- ^ S. Breton: The cellular physiology of carbonic anhydrases . In: JOP: Journal of the Pancreas . tape 2 , 4 Suppl, June 2001, pp. 159-164 , PMID 11875253 ( HTML full text [accessed 12 February 2011]).
- ↑ P. Hescheler (ed.); Speckmann Deetjen: Physiology . Urban & Fischer in Elsevier, Munich 2006, ISBN 978-3-437-44440-1 , pp. 591-632.
- ↑ Philip R. Debruyne, Erik A. Bruyneel, Xuedong Li, Amazia Zimber, Christian Gespach, Marc M. Mareel: The role of bile acids in carcinogenesis . In: Mutation Research / Fundamental and Molecular Mechanisms of Mutagenesis . tape 480-481 , August 1, 2001, pp. 359-369 , doi : 10.1016 / S0027-5107 (01) 00195-6 , PMID 11506828 .
- ↑ C. Baur et al .: Neutralizing capacity, pepsin inactivation and binding to bile acids and lysolecithin of the antacid magaldrate Arzneimittelforschung 1981; 31 (3): 504-7, PMID 6784737 .
- ↑ LE. Borella et al .: Cytoprotective and antiulcer activities of the antacid magaldrate in the rat. Drug Research 1989 Jul; 39 (7): 786-9, PMID 2783181 .
- ↑ Patent US2923660 : Process for the preparation of magnesium aluminate hydrate, and therapeutic agents so produced. Applied on July 17, 1956 , published on February 2, 1960 , applicant: Byk Gulden Lomberg Chem. Fab., Inventor: Gunther Hallmann.
- ↑ Technical information Riopan gastric tablets, as of December 2014.
- ↑ Dr. Kade buys Riopan and Faktu . Apotheke adhoc, March 28, 2013.