Foot and mouth disease
| Classification according to ICD-10 | |
|---|---|
| B08.8 | Other specified viral infections characterized by skin and mucous membrane lesions |
| ICD-10 online (WHO version 2019) | |
The foot-and-mouth disease (FMD), also Aphthenseuche , aphthae epizooticae and stomatitis epidemic , is a highly contagious viral disease in cattle and pigs and is a notifiable animal disease . Other ungulates such as deer , goats and sheep , but also elephants , rats and hedgehogs can become infected . Horses are not, and humans are rarely, susceptible to FMD.

Pathogen
In 1898, Friedrich Loeffler and Paul Frosch were the first animal virus to prove that the FMD pathogen was viral. The two bacteriologists discovered that intravenously administered lymph from infected animals was the cause of disease for healthy calves even after prior filtration through bacteria- proof diatomite . Foot -and-mouth disease virus is the causative agent of FMD , a highly contagious single (+) strand RNA virus [ss (+) RNA]. It belongs to the genus aphthovirus of the virus family Picornaviridae . The members of this family are non-enveloped small (25-30 nm) viruses with an icosahedral capsid (protein shell), which contains single-stranded ribonucleic acid (RNA) as genetic viral material . After the protein envelope has dissolved, virus replication takes place in the cytoplasm of an infected host cell. The newly formed virions are released after the cell membrane has dissolved by lysis .
transmission
In contrast to other pathogens, researchers believe it is possible with the foot-and-mouth disease virus that, at least in theory, a single virion of this pathogen can trigger an infection .
Foot-and-mouth disease caused by infection with the virus usually occurs locally and the virus is primarily transmitted through contact and smear infection through direct contact with infected animals, contaminated pens or cattle transport vehicles. However, the virus can also be spread through the air. The clothing and skin of farmers and other people around animals, standing water, uncooked feed waste, feed additives containing infected animal products, and animal products such as cheese or meat can harbor the virus. Cows can get foot-and-mouth disease from infected bulls through semen transmission . Control measures include quarantine , the destruction of infected cattle herds and an export ban on animal products to countries not affected by the disease.
People cannot become infected with foot and mouth disease. For consumers of beef and pork as well as pasteurized milk or products made from them, there is no danger even in the event of an epidemic. However, as the disease spreads extremely quickly among animals, FMD is a serious threat to agriculture.
Occurrence
FMD is widespread almost worldwide. Only in New Zealand have no foot-and-mouth disease outbreaks been registered; in Australia the last outbreak was in 1872. The United States (last occurrence in 1929), Canada (1952), Mexico (1954) and Chile (1988) are also considered to be FMD-free. In Europe, only the Scandinavian countries of Norway (last occurrence in 1952), Finland (1959) and Sweden (1966) have been spared from outbreaks in recent decades . The last epidemic occurred in Austria in 1973. The disease last occurred in Germany in 1988. There were cases of FMD in 2001 in Spain, France, the Netherlands and Ireland. Outbreaks of FMD were last seen in Great Britain in 2001 and 2007. In Switzerland the epidemic occurred in the years 1871/72, 1899–1900, 1911–14, 1920/21, 1939/40 and 1965 with devastating effects. After the last outbreaks in 1968 and 1980, Switzerland is officially free of FMD.
FMD is still widespread in Africa , Asia and parts of South America. These regions are considered enzootic . In Europe, the disease is largely under control through state veterinary surveillance, but it is always introduced. The diverse transmission routes and the rapid rate of spread of FMD quickly lead to epizootics, so that the epidemic is a constant threat to Europe.
Pathogenesis
The focus of the infection is a strongly pronounced viraemia phase, with which the generalized spread of the pathogen in the host and its settlement in the target organs are associated. The FMD virus has a high affinity for the skin and cutaneous mucous membranes ( epitheliotropism ). Affected are the mucous membranes of the oral cavity, esophagus and rumen pillars as well as the hairless skin of the nostrils, muzzle, proboscis, udder and claws. The pathogen also affects the skeletal and heart muscles ( myotropism ). Neurotropic properties are rarely observed.
Cattle are mainly infected by air , while infection in pigs usually occurs via the oral route. The aphtha ("primary aphthous") arising at the entrance gate usually escapes observation. From the primary place of multiplication (pigs: tonsils, cattle: pharyngeal mucosa and bronchioles), the virus reaches the lymphoreticular system (especially liver and spleen) in an initial viraemic phase via lymph and blood. The course of the disease is determined by the further success of the virus in multiplying in the primarily affine organs. In the case of strong multiplication, colliquational necrosis occurs there , followed by generalized viraemia, in the course of which the FMD pathogen reaches the muscles, skin, mucous membranes and occasionally the CNS. The viraemia lasts four days. After generalization, the viral RNA is widely detectable in various epithelia. A visible sign of the organ manifestation of the virus is the formation of "secondary naphtha". Predisposing factors such as particular mechanical stress favor their development. The formation of aphthae takes place in the stratum spinosum of the epidermis : the infected keratocytes are destroyed and the resulting cavities fill with clear fluid that confluences into a large bladder. The base of the bladder is formed by the intact stratum basale with the underlying, well-perfused papillary body . After bursting, the aphthae have a tendency to often flat erosions. Depending on the myotropic affinity of the pathogen strain, cell damage of variable extent occurs in heart and muscle cells.
Clinical symptoms and course of disease
Beef
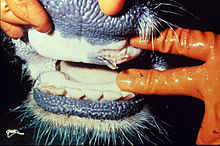
The incubation period is two to seven days. The outbreak of the epidemic is characterized by a fever phase of up to three days (up to 42 degrees Celsius), which is associated with severe disorders of the general condition. In dairy cows, a sudden drop in milk yield up to the point where the milk runs dry can be observed. Even in the fever phase, a strong production of viscous saliva ("MKS beard") begins with simultaneous reddening of the oral mucosa. Loss of appetite, rumination disorders and the appearance of "saliva pools" in the vicinity of animals characterize the progression of the disease.
As the disease progresses, fluid-filled blisters ( aphthae ) the size of peas and pigeons' eggs form on the mouth of the mouth , in the entire mucous membrane of the mouth and in the tongue area . At the same time, other aphthae develop in the claw area, on the udder skin and on the teats. The general condition is severely disturbed. The animals show expressions of pain in the form of closed mouths, smacking jaw movements and lameness. After the blisters burst, erosions - in some cases large areas - form and the healing process begins. At the same time, other animals in the herd are constantly falling ill. Healing of the lesions in the mouth area takes up to 14 days. The claw naphtha heals within a month. The convalescence phase is often disturbed by secondary bacterial infections.
In addition to benign disease variants with partially mild symptoms ( mortality 2–5%), there is also a malignant form of the disease (mortality up to 80%). The cause is strongly virulent pathogens with pronounced myotropism. This form occurs preferentially in calves. Even with mild courses, heart muscle damage caused by myocarditis is in the foreground in this age group . Affected animals die within 24 hours with the symptoms of a severe general illness.
The natural infection leaves a resilient immunity against the respective virus type for up to 12 months. Dreaded and expected consequential and long-term damage after surviving an infection are bacterial secondary infections, mastitis , sole horn and claw changes up to " shoing " (complete detachment of the claw horn), muscle damage, myocarditis as well as persistent massive depression and loss of fitness.
The most important differential diagnoses in cattle include vesicular stomatitis , mucosal disease , rinderpest , malignant catarrhal fever , infectious bovine rhinotracheitis and smallpox .
pig
The incubation period is one to three days (in individual cases up to a maximum of 12). The initial feverish phase lasts for up to four days. Overall, the epidemic is less dramatic than in cattle.
The claws are primarily affected by the aphthous ulcer, which is mainly visible on the coronet and in the gap between the claws. The changes to the proboscis and oral mucosa are rather inconspicuous. In the sow there are also sucking phthens.
The clinical picture of the older animals is characterized by (supporting leg) lameness of varying degrees of severity up to the fact that they are stuck. Initially only a few animals are affected, the disease spreads within a few days in the herd. Sudden deaths occur in suckling pigs and runners due to heart muscle damage. The epithelial lesions on the proboscis and teats heal within two weeks, with coronary and sole defects, the course of the disease is usually made more difficult by purulent secondary infections.
Post-infection immunity lasts for five to seven months. Late damage and consequential damage include mastitis, metritis and abortions, shoing of the animals and loss of performance caused by myocarditis.
Further diseases of the vesicular disease complex in pigs cannot be distinguished from the clinical picture, all of which are associated with vesicular formation: vesicular stomatitis (VS), swine vesicular disease (SVD) and vesicular rash (VES). Selenium poisoning is also an option for differential diagnosis .
Sheep and goat
The incubation period of the so-called "benign mouth disease" is 2–14 days. In sheep and goats, the signs of illness are less noticeable than in cattle.
In sheep, the focus is on the formation of aphthae on the coronet and in the gap between the claws. Changes to the oral mucosa and lips are often uncharacteristic. Infestation of the herd is slow and incomplete (within three to six weeks). Often the only signs of the disease are painful and severe lameness. From the third day of lameness, the reddened sores become clearly visible after the aphthae have burst. The mortality of the adult animals is low. In lambs, the malignant myocarditic form dominates with fever, diarrhea and apathy. The losses are up to 80%; the animals perish without any signs of aphthous ulcer formation. The lesions heal within two to three weeks, and secondary infections complicate the course.
In goats , the disease is either mild or severe, combined with myocardial damage and high mortality. There is a febrile phase with general disturbances and a decline in milk. The formation of aphthae in the mucous membrane of the mouth is clear, but if it ruptures soon, it only lasts for a short time. In the head area there can be swelling with standing up of the hair (so-called dickkop ). Often there is rhinitis. Claw naphtha is rarely seen.
Late and consequential damages in small ruminants are hoof infections, abortions, metritis and mastitis. The type-specific immunity after field virus infection is one to two years and longer.
In sheep and goats, changes similar to those caused by foot-and-mouth disease are caused by the mustache , lip rash (Ecthyma contagiosum), sheep and goat pox .
human
Due to their low susceptibility, people are affected by the disease only extremely rarely and the prognosis for diseases is favorable. The infection occurs directly through contact with infected animals or as a result of a laboratory infection. Indirect transmission via infected milk is also possible.
With an incubation period of two to six days, the disease proceeds as a biphasic-cyclic infection, just as it does in cloven-hoofed animals. After a short, moderate phase of fever and unspecific general symptoms such as nausea, exhaustion, headache and body aches, painful aphthae can appear in the reddened oral mucosa , but preferably on the skin of the hands (fingertips) and feet and in the genital area. The skin erosions that develop after the aphthae have dried out usually heal completely within ten days.
In terms of differential diagnosis, the disease, which is commonly referred to as hand, foot, and mouth rash , which is also viral and is associated with very similar symptoms , must be distinguished . It is described more often in humans, especially young children. This disease is of a different virus from the family of Picornaviridae, the enterovirus Coxsackie A caused.
Pathological-anatomical findings
In addition to the externally visible changes, there are other aphthae of various healing stages in the throat and esophagus. On rumen pillars and Psalterblättern only schorfbedeckte lesions are often visible. In the absence of aphthous ulcers in the mucous membranes, catarrhal swellings or minor bleeding appear. Petechial bleeding may also be visible under the epicardium . No lesions can be observed in the myocardium and skeletal muscles of older animals and the virus only appears to multiply in these locations in young animals. The high mortality in young animals is thought to be due to acute myocarditis, which can be seen to the naked eye by venous congestion and large blood clots , v. a. in the left ventricle. The damaged heart muscles are soft, poorly contracted and show gray-white stripes and spots of variable sizes (“tiger heart”). The left ventricle and cardiac septum are particularly affected . This form of the disease is often accompanied by the complete absence of aphthous changes in the usual predilection sites. In the case of a peracute course, there may even be no visible changes in the heart muscle. Occasionally the skeletal muscles are also affected by streaky changes.
Histological picture
In the early stages, the lesions can only be made visible under the microscope . The first histological changes in the stratum spinosum are characterized by vacuolar degeneration, increasing eosinophilic staining of the cell cytoplasm and the formation of edema in the intercellular space. This is followed by cell necrosis and reactive infiltration with leukocytes ( monocytes , granulocytes ). The so-called aphthous ulcers develop from the lesions that have become visible in the meantime, as the epithelium is separated from the underlying tissue and the cavity is filled with clear, vesicular fluid. In some cases the amount of fluid can be minimal. The epithelium can also become necrotic or tear apart from mechanical trauma without aphthous ulcers taking place. The histological picture of lymphohistiocytic myocarditis with hyaline- lumpy degeneration (“Zenker's muscle degeneration”) and necrosis of the heart muscle cells (myocytes) arises in the heart muscle.
Diagnosis
If FMD is suspected, the actual outbreak must be officially established. According to Section 1 of the “Ordinance on Protection against Foot and Mouth Disease” (FMD-VO), an outbreak of the disease is only considered proven when the pathogen is detected in the form of virus antigen or viral RNA . This also applies in the case of missing clinical symptoms. In addition, the serological detection of antibodies against FMD or a titer increase in animals that have been proven not to be vaccinated is binding. If there is an epizootiological connection to a primary epidemic focus ( secondary outbreak ), the results of clinical or pathological - anatomical examinations alone may be sufficient.
On-site measures
In cattle, the clinical picture is usually clear. The diagnosis of small ruminants is often made difficult by inapparent and mild forms. The occurrence of sudden lameness of most of the herd at the same time increased peracute mortality of newborn and / or very young lambs are the first evidence of FMD before the pathognomonic evidence of Aphthe nbildung. In pig herds, differential diagnosis of foot-and-mouth disease must be considered as soon as there is frequent lameness in connection with blisters in areas predisposed to aphthous ulcers.
A thorough examination of the population under suitable conditions (good lighting, mechanical cleaning of soiled predilection sites ) in the event of suspicion, precise knowledge of the clinical picture and a veterinarian who is sufficiently sensitized to the epidemic are prerequisites for rapid detection of the disease.
FMD is a notifiable animal disease. In the event of suspicion, the animal owner, the nursing staff or the practical veterinarian must immediately consult the official veterinarian. This examines the herd, if necessary takes samples for further laboratory diagnostic clarification and makes additional initial animal health orders in accordance with the FMD Regulation (see under measures).
The samples taken must be forwarded to the national FMD reference laboratory as quickly as possible (by courier or helicopter) without any loss of time . Suspicious samples are to be announced to the investigation facility in advance so that immediate further processing can be ensured.
For the detection of infectious virus, antigen or nucleic acid , lymph and cover material from fresh aphthous ulcers are best suited. If there are no aphthae, swabs can alternatively be removed from the transition to the healthy tissue. In addition, nasal swabs as well as organ samples from killed animals (e.g. changed areas of rumen pillars, heart and udder) can be used as sample material. The samples are mixed with PBS buffer and glycerine and sent cooled at a neutral pH value. If more than a week has passed since the infection, the virus detection from throat mucus samples (so-called "probang" samples) replaces the swab removal from the nose. The dispatch of these samples must be frozen. Blood samples not only contain virus antibodies (from the 5th day after infection), but are also used for virus detection in the viremic phase. The additional submission of serum samples is mandatory, especially for small ruminants .
Laboratory diagnostic procedures
Approved detection methods in FMD diagnostics as well as reference laboratory protocols for carrying out the test can be found in the "Manual of Diagnostic Tests and Vaccines for Terrestrial Animals"; Chapter 2.1.1. (Foot and mouth disease) from the OIE . At the national level, information on the diagnosis of foot-and-mouth disease is set out in the "Federal Catalog of Measures for Animal Diseases", Part III.2.
The FMD diagnostics may only be carried out by high security laboratories of security level 4 due to the high level of pathogen contagion. The German FMD reference laboratory is part of the Friedrich Loeffler Institute . The German research facility is located on the island of Riems . The international reference laboratory is located at the Institute of Animal Health in Pirbright , England.
The most urgent task of laboratory diagnostics is the determination of a primary outbreak in order to be able to initiate the culling of infected stocks and the establishment of blocking measures as quickly as possible . Once the suspicion has been confirmed, the virus is characterized so that a possible vaccine recommendation can be made. In addition, epidemiological studies on a molecular genetic basis are carried out in order to determine the geographical origin of the pathogen (“tracing back”). The laboratory diagnostic methods are divided into pathogen detection and antibody detection.
Pathogen detection
The detection of viral antigens or viral nucleic acids of the FMD pathogen is sufficient for a positive test result.
The preferred method for virus antigen detection with simultaneous determination of the serotype is the enzyme-linked immunosorbent assay ( ELISA ). Due to its 10–100 times higher sensitivity (better sensitivity and specificity) and lower susceptibility to interference, it has replaced the classic complement fixation reaction (KBR) as a fast detection method (<1 day). It is carried out as an indirect, so-called "double sandwich ELISA". Rabbit antibodies bound to the microtiter test plate hold the virus antigen to be detected. After a subsequent incubation with guinea pig antibodies, the antigen-antibody reaction by adding a peroxidase - conjugate and a specific substrate made optically visible. The eight rows of the microtiter plate each contain antisera against the seven different known FMD serotypes. The ELISA thus allows a preliminary type diagnosis, which, however, requires further confirmation with monoclonal antibodies for safety . In the free eighth row, differential diagnostics can be used to investigate further viral pathogens (e.g. pig vesicular disease ). Questionable results can be checked again in the repeated ELISA run after the sample material has passed through cell culture.
Only 80% of all samples that are positive in the cell culture show a reaction in the ELISA. Due to the serious consequences of a confirmed suspicion, virus isolation by means of cell culture is always used in Germany in parallel with the ELISA. Infectious sample material from suspected clinical cases is inoculated into susceptible cell cultures (e.g. calf thyroid cells, hamster kidney cells) or into baby mice two to seven days old in order to multiply potential infectious virus. At least one to three days pass before a cytopathogenic effect occurs or the test animal dies. A subsequent virus replication phase is followed by identification and characterization using ELISA or reverse transcriptase polymerase chain reaction (RT-PCR).
In order to have a second laboratory method available with a sensitivity comparable to that of cell culture, tests for the detection of viral nucleic acid have been established. RT-PCR can be used to detect genome fragments of the FMD virus from a wide variety of diagnostic materials. In combination with real-time quantitative PCR , a sensitivity can be achieved that is comparable to virus isolation. Process steps that can be automated enable a higher sample throughput. The classic PCRs were used for type-independent FMD detection by selecting a genome region that was largely conserved for all serotypes for the amplification . However, the use of specific primers also allows reliable differentiation of the seven serotypes. The detection of viral RNA in tissue samples is also possible by means of in situ hybridization . This technique is only used by a few specialized laboratories, although simplified test systems for “field use” are already in the development phase.
The virus genome is also used for molecular epidemiology research. The basis is the comparison of genetic differences between individual virus variants. Based on the sequencing data of the 1D gene (encoded for the viral envelope protein VP1), family trees have now been created that show the genetic relationship between vaccine and field virus strains of all seven serotypes. Many laboratories have developed their own methods here. The amplification of the viral RNA using RT-PCR, followed by the decoding of the nucleotide sequence (sequencing) is usually the method of choice for generating this data. The databases of the reference laboratories now contain more than 3000 partial sequences.
Antibody detection
Serological tests for FMD are mainly used to clarify the following questions: a) Certification for the export of certain animals as free of FMD infection and vaccination; b). Confirmation of suspected disease cases; c). Evidence of the absence of infection and d). Confirmation of the effectiveness of a vaccination. Serological detection methods for FMD antibodies (Ab) are divided into those that detect immunoglobulins against structural proteins (SP) and those that detect antibodies directed against non-structural proteins (NSP).
Detection of structural protein antibodies
The virus neutralization test and various types of antibody ELISAs ("solid-phase competition ELISA" (SPCE); "liquid-phase blocking ELISA" (LPBE)) for the detection of structural proteins are used as serotype-specific serological tests. They are mandatory in the international pet trade. They recognize antibodies that are produced in response to vaccination as well as natural FMD infection. As a result, they are useful for detecting recent infections in non-vaccinated individuals or for monitoring the immune status of a population in vaccination programs.
As a highly sensitive method, they require a strong similarity between the circulating field strain and the FMD virus antigen used in the test. Using poly- or monoclonal AK, the ELISA tests are versatile, quick to perform and can also be prepared with inactivated antigens. False positive reactions in the ELISA are to be expected with a small percentage of samples in the low titer range. The virus neutralization test depends on the use of cell cultures and is therefore less versatile. It also lasts longer (four days) and is more susceptible to contamination. Combined use of the ELISA as a screening method with subsequent testing of reagents in the VNT reduces the probability of false positive results to a minimum.
Detection of non-structural protein antibodies
Assays for antibodies to non-structural proteins are useful in detecting recent or ongoing virus replication in the host, regardless of its vaccination status. In contrast to structural proteins, non-structural proteins are highly conserved within the FMD virus species . The detection of these antibodies is consequently not serotype-specific, which is an advantage as a detection method on an international level.
The traditional test for the detection of FMD-NSP was the immunodiffusion test . It was used to detect “virus infection associated antigen” (VIAA) with the main component Protein 3D. However, the latter can also be formed by vaccinated individuals.
Modern processes use the genetically engineered proteins 3ABC and 3AB. Antibodies against these proteins are generally seen as reliable indicators of an FMD infection, since they show a high experimental immunogenicity . Antibodies against expressed recombinant viral NSP (e.g. 3A, 3B, 2B, 2C, 3ABC) can be detected by various ELISA variants or by immunoblotting . Three indirect ELISA methods are approved for FMD (South America, Denmark, Brescia). These ELISAs either use purified antigens that are bound directly to the test plate or use polyclonal / monoclonal antibodies to “capture” specific antigens from semi-purified preparations.
The enzyme-linked immunoelectrotransfer blot assay (EITB) was first published in 1993. It is widely used in South America, where it is also used as a confirmatory test for reagents found in ELISA screening.
The methods have a specificity of 99%. However, a lack of purity of the vaccines can adversely affect the diagnostic specificity. The presence of NSP in some vaccine preparations can lead to incorrect assignment of animals that have already been vaccinated repeatedly.
The test sensitivity is considered unsatisfactory in experimentally vaccinated cattle that have been exposed to a field virus infection and subsequently showed virus persistence (vaccinated "carriers"). They only show a local immune response, but not immunoglobulins G against NSP. However, it appears that carrier animals can be detected using Ig A from saliva. These antibodies are evidently only produced by actually infected animals over a longer period of time.
Control measures

Until December 31, 1991, compulsory vaccinations of cattle were carried out in the EU to prevent an FMD epidemic . Vaccinations lead to serious barriers to trade: like infected animals, vaccinated people have antibodies in their blood and can therefore only be differentiated from each other with specially marked vaccines . There is also the risk of the pathogen spreading through vaccinated animals. Therefore vaccinations by the EU were stopped. Therapy measures are also generally not permitted.
If FMD is suspected, the farm in question is closed, sheep are usually culled as a precaution and samples are examined for FMD. Furthermore, a restricted area with a radius of at least three kilometers will be set up, all animal populations will be examined for FMD and animal transport will be prohibited.
If the suspicion is confirmed, the population is culled and disposed of harmlessly, as are the neighboring populations within a radius of one kilometer. In the three-kilometer restricted area, no animals or sperm may be transported for 15 days , the roads will be closed. After these 15 days, animals may only be transported with a permit (animals may only be transported for slaughter). Milk may only be used for separate processing. An observation area will be set up within a radius of 10 km from the outbreak. There animals can be transported within the area with a permit. If no further illnesses have occurred within 30 days of the epidemic, rats and mice are controlled, as well as cleaning and disinfection.
Since the virus is very resilient, it can persist for months in the ground, stalls, waste and straw. In the event of an infestation, extensive disinfection with formic acid or heat (at least 60 ° C) must therefore be carried out.
Measures such as a ban on animal transport are often taken when an FMD epidemic occurs in neighboring countries. Because of its high resistance, even the wheels of cars are disinfected when crossing the border in the event of major epidemics.
Epizootia
Europe was often affected by FMD epizooties or even panzootias . There were particularly severe epidemics in 1910–1912, 1919–1921, 1937–1939 and 1950–1952.
In the UK in February 2001 broke out epizootic. During this outbreak, which spread sporadically to mainland Europe, it came to the culling of more than four million animals. It was not until January 14, 2002, after three months without reports of new cases, that the island was declared free of the disease again.
There are always isolated outbreaks in the rest of Europe, Africa, Asia and South America, as most recently on August 3, 2007 in Surrey (Great Britain).
literature
- Wolfgang Bisping: Compendium of the state control of animal diseases. Stuttgart: Enke 1999, ISBN 3-7773-1423-4 , pp. 101-104
- Hans Plonait, Klaus Bickhardt (Hrsg.): Textbook of pig diseases . 2., rework. Edition Berlin: Parey 1997, ISBN 3-8263-3149-4 , pp. 66-68
- Hartwig Bostedt; Kurt Dedié: Sheep and Goat Diseases . 2., rework. and exp. Aufl. Stuttgart: Ulmer 1996, ISBN 3-8252-8008-X , pp. 35–37 (diseases of domestic animals; UTB for science: large series)
- Gustav Rosenberger (Ed.): Diseases of the cattle. 3rd, unchanged. Edition Berlin [u. a.]: Blackwell-Wiss.-Verl. 1994, ISBN 3-8263-3029-3 , pp. 835-843 (Blackwell Science)
- Winfried Hofmann, Hartwig Bostedt: cattle diseases. Vol. 1: Internal and surgical diseases. Stuttgart: Ulmer 1992, ISBN 3-8252-8044-6 , p. 243f. (Diseases of pets)
- Anton Mayr (Ed.): Rolle / Mayr. Medical microbiology, infection and epidemic science for veterinarians, biologists and agricultural scientists and those interested in related fields: textbook for practice and study. 6., rework. Aufl. Stuttgart: Enke 1993, ISBN 3-432-84686-X , pp. 311-317.
- Animal diseases in the tropics and subtropics . Ed. by the British Veterinary Association. Konstanz: Terra-Verlag 1968, pp. 51-57.
- Joachim Beer: Foot and Mouth Disease. In: J. Beer (ed.): Infectious diseases of domestic animals. Jena: Fischer-Verlag 1974.
Individual evidence
- ↑ Karl Wurm, AM Walter: Infectious Diseases. In: Ludwig Heilmeyer (ed.): Textbook of internal medicine. Springer-Verlag, Berlin / Göttingen / Heidelberg 1955; 2nd edition, ibid. 1961, pp. 9-223, here: pp. 209 f.
- ↑ FLI : Foot and Mouth Disease: Official method and case definition
- ↑ Daniel Baumann, Daniel Freudenreich: One virus particle is enough for an infection. In: Berliner Zeitung . August 7, 2007, accessed June 19, 2015 .
- ↑ FLI : Foot and Mouth Disease: Official method and case definition
- ↑ NÖN : 3,732 cattle had to be culled , week 32/2013
- ↑ Animal Disease Report 2011 by the BMELV . In: Deutsches Tierärzteblatt. (DTBL) Volume 60, May 2012, pp. 714–715.
- ^ ADNS (Animal Disease Notification System): Animal disease situation per country and per disease, different years.
- ↑ Article cattle diseases in the Historical Lexicon of Switzerland
- ↑ Foot and Mouth Disease
- ↑ Swiss Association for the History of Veterinary Medicine SVGVM: Fighting Foot and Mouth Disease in the 20th Century ( Memento of the original from January 1, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Keystone: Foot and Mouth Disease (1965/1966) ( Memento of the original dated November 30, 2018 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ OIE : OIE Members' official FMD status map
- ↑ Stern, August 6, 2007: From the laboratory to the stable.Retrieved : December 25, 2014
Web links
- International FMD reference laboratory
- Flyer of the Friedrich Löffler Institute (PDF, 2 pages, 3.96 MB)