Nicotinic acid
| Structural formula | |||||||||
|---|---|---|---|---|---|---|---|---|---|

|
|||||||||
| General | |||||||||
| Common name |
|
||||||||
| other names |
|
||||||||
| Molecular formula | C 6 H 5 NO 2 | ||||||||
| CAS number | 59-67-6 | ||||||||
| PubChem | 938 | ||||||||
| ATC code | |||||||||
| DrugBank | DB00627 | ||||||||
| Brief description | colorless crystals | ||||||||
| Occurrence | Poultry, liver, coffee, brewer's yeast | ||||||||
| physiology | |||||||||
| function | Part of the coenzymes NADH and NADPH | ||||||||
| Daily need | 15-20 mg | ||||||||
| Consequences in case of deficiency | mild: irritability, loss of appetite, concentration and sleep disorders severe: pellagra |
||||||||
| Overdose | about 1.5-3 g per day | ||||||||
| properties | |||||||||
| Molar mass | 123.11 g mol −1 | ||||||||
| Physical state | firmly | ||||||||
| density | 1.47 g cm −3 | ||||||||
| Melting point |
236.6 ° C |
||||||||
| pK s value | * pK s N (25 ° C) = 4.82
|
||||||||
| solubility | little in water (18 g l −1 at 20 ° C) | ||||||||
| safety instructions | |||||||||
|
|||||||||
| Toxicological data | |||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||
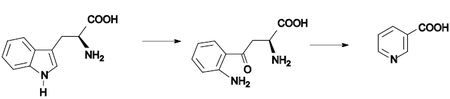
Nicotinic acid (also: niacin ) is a vitamin from the B complex . The designation vitamin B 3 , more rarely vitamin PP or PP factor ( Pellagra -Preventing-Factor) for nicotinic acid, is now considered outdated and outdated. Nicotinic acid was discovered in 1867 during the oxidation of nicotine ; its physiological effectiveness was recognized in 1934. Nicotinic acid ( pyridine-3-carboxylic acid ) is an organic compound that belongs to the group of heterocycles (more precisely: heteroaromatic compounds ). It consists of a pyridine ring which is substituted with a carboxy group (−COOH). Together with the other two isomers, picolinic acid and isonicotinic acid, it belongs to the group of pyridinecarboxylic acids with the empirical formula C 6 H 5 NO 2 .
history
Nicotinic acid was first synthesized in 1867 by C. Huber. He used nicotine and let it oxidize. The physiological significance was not investigated until years later (1914). Russell Henry Chittenden and Frank Pell Underhill (1877–1932) recognized in 1917 that nicotinic acid was involved in the vitamin system. Paul Jones Fouts (* 1905) was not able to prove the exact connection between Pellagra's disease and a nicotinic acid deficiency until 1937. Nicotinic acid was then used as a dietary supplement. Attempts were later made to further develop the derivatives by producing various nicotinic acid esters. The butyl ester was found to be the most effective. Initially, both substances were used topically for the treatment of rheumatic pain. Later nicotinic acid (Niconacid®) and nicotinyl alcohol (Ronicol®) were used for the oral therapy of arterial peripheral circulatory disorders.
Occurrence / properties
Nicotinic acid is found in all living cells and is stored in the liver. It forms an important component of various coenzymes ( NAD , NADP ) and in this form is of central importance for the metabolism of proteins , fats and carbohydrates . Nicotinic acid is less sensitive to heat, light and atmospheric oxygen than other vitamins of the B group .
Nicotinic acid is a crystalline solid. It occurs in two polymorphic forms. Crystal form II is present at room temperature. When heated to 178.8 ° C., a weakly endothermic solid phase transition with Δ fus H = 0.81 kJ / mol to crystal form I is observed. This then melts at 236.6 ° C with a melting enthalpy of Δ fus H = 27.57 kJ / mol.
From the nicotinic acid from one another in the forwards Di nicotinic acid ( pyridine-3,5-dicarboxylic acid ), which at the pyridine axisymmetric two carries carboxyl groups.
Synthesis / manufacture
Nicotinic acid is formed by the oxidation of nicotine with nitric acid . Alternatively, it can be prepared from quinoline by oxidation using potassium permanganate (KMnO 4 ), whereby quinolinic acid is formed as an intermediate :
Finally, nicotinic acid is also obtained by oxidizing 3-picoline with potassium permanganate:
Only the oxidation of 5-ethyl-2-methyl-pyridine (MEP) using nitric acid is of importance today.
biosynthesis
Little is known about the biosynthesis of nicotinic acid in fungi and plants, especially about the enzymes involved. The oxidative breakdown of tryptophan via kynurenine to nicotinic acid is probably the most common.
However, this path does not play a role in humans, since the actual purpose of niacin, the biosynthesis of NAD, also works with the tryptophan breakdown product quinolinic acid .
Task / function
Nicotinic acid is involved in the protein, fat and carbohydrate metabolism. In the form of the coenzymes NAD / NADP and their reduced forms NADH + H + / NADPH + H + , nicotinic acid is used as a hydrogen carrier, i.e. a reducing agent, e.g. B. involved in the citric acid cycle and the respiratory chain . It has an antioxidant effect and takes part in many enzymatic processes. Nicotinic acid is important for the regeneration of skin , muscles , nerves and DNA .
Occurrence and need
Natural sources of nicotinic acid are foods such as poultry , game , fish , mushrooms , dairy products and eggs . Also liver , coffee , cashew nuts , whole grains , various vegetables and fruits contain nicotinic acid, being recycled from animal products fundamentally better by the body. Vegans can get their needs from peanuts , wheat bran , dates , mushrooms , brewer's yeast , dried apricots and legumes , for example .
The body's daily need for nicotinic acid depends on its energy needs. On average, the adult body needs about 6.6 milligrams of niacin to produce 1,000 kilocalories of energy for its cells, tissues and organs. The requirement for women is 13 to 15 mg of nicotinic acid per day, for men 15 to 20 mg per day. 5 to 6 mg is recommended for children and around 20 mg for breastfeeding women.
In dairy cows, niacin is used as a feed additive. Here it ensures a more balanced energy balance.
Deficiency symptoms (hypovitaminosis)
Symptoms of deficiency rarely occur because the body can produce NAD not only from niacin, but also from the amino acid tryptophan . A low-protein diet or absorption disorders can initially lead to unspecific disorders such as loss of appetite, concentration and sleep disorders and a certain irritability. Symptoms of nicotinic acid deficiency are still:
- Skin changes ( dermatitis )
- diarrhea
- depressions
- Inflammation of the mouth and gastrointestinal mucous membranes
- Disease: Pellagra (can lead to dementia, among other things)
The appearance of Pellagra's disease is related to the introduction of low-tryptophan corn outside of Central America. In its country of origin, Mexico, the maize is still usually placed in alkaline lime water after harvesting and ground wet, which is what releases the nicotinic acid in the maize. The Spanish conquerors brought maize to southern Europe, North America and Africa without adopting this preparation technique there. Because of the higher yields, many farmers soon switched from wheat and barley to maize. As a result, entire sections of the population for whom maize was the main source of food developed nicotinic acid and tryptophan deficiency symptoms. It was not until the beginning of the 20th century that the connection between pellagra and corn nutrition was clarified.
Consequences of an overdose (hypervitaminosis)
One speaks of an overdose of nicotinic acid at a dose of 1.5–3 g per day. With a supply of about 500 mg per day, in some cases even less, it comes to hautgefäßerweiternden effect flush and at a level of about 2500 mg per day can drop in blood pressure , dizziness and increased uric acid in the blood occur. Ingesting high doses of several grams can cause diarrhea, nausea, vomiting and liver damage which manifests as jaundice .
Nicotinic acid as a medicinal substance
Nicotinic acid lowers LDL cholesterol , Lp (a) and triglycerides and increases HDL cholesterol . However, despite the changed blood lipid levels, no medical benefit could be demonstrated. A Cochrane - meta-analysis showed that nicotinic acid in a median dose of 2 g per day (in adult humans) no deaths or cardiovascular disease significantly reduced, but leads to an increased risk of diabetes by 30%.
The most common side effect is flushing symptoms , which can limit therapy. However, it can be counteracted with acetylsalicylic acid or laropiprant , as these two active ingredients prevent the vasodilating effect of the prostaglandins that play a role in this. Gastrointestinal complaints are also common. Prolonged use of high-dose nicotinic acid preparations can worsen glucose tolerance and increase uric acid levels in the blood. Nicotinyl alcohol, which is derived from nicotinic acid, also has an effect on blood lipid levels .
The National Institutes of Health (NIH) of the United States stopped end of May 2011 a large-scale study of over 3400 patients to the parallel lipid-lowering simvastatin nicotinic acid-release tablets (Niaspan) received. Niaspan was unable to reduce the rate of heart attacks. In contrast, the number of strokes increased slightly in the patients who also received Niaspan . In July 2011, sales of Niaspan were discontinued; the successor product Tredaptive , a combination of nicotinic acid with laropiprant, could not hold its own in the market due to an unfavorable risk-benefit ratio.
See also
Individual evidence
- ↑ entry to NIACIN in CosIng database of the European Commission, accessed on February 17 2020th
- ↑ Entry on nicotinic acid. In: Römpp Online . Georg Thieme Verlag, accessed on June 26, 2014.
- ↑ a b c d e Entry on nicotinic acid in the GESTIS substance database of the IFA , accessed on April 27, 2017(JavaScript required) .
- ↑ a b Zvi Rappoport (Ed.): CRC Handbook of Tables for Organic Compound Identification . 3 rd Edition, CRC Press / Taylor and Francis, Boca Raton, FL, 1967, ISBN 0-8493-0303-6 , Acid Dissociation Constants of Organic Acids in Aqueous Solution, S. 432nd
- ^ Entry on nicotinic acid in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ^ LF Fieser, M. Fieser: Organic chemistry. Verlag Chemie, Weinheim 1965, pp. 1675–1676.
- ↑ JG Wooley, WH Sebrell: "Niacin (Nicotinic Acid) to Essential Growth Factor for Rabbits [...]", Division of Physiology, National Institute of Health, US Public Health Service, Bethesda, Maryland, 1944th
- ^ Wolf-Dieter Müller-Jahncke , Christoph Friedrich , Ulrich Meyer: Medicinal history . 2., revised. and exp. Ed. Wiss. Verl.-Ges, Stuttgart 2005, ISBN 978-3-8047-2113-5 , pp. 165 .
- ^ SX Wang, ZC Tan, YY Di, F. Xu, MH Wang, LX Sun, T. Zhang: Calorimetric study and thermal analysis of crystalline nicotinic acid. In: J. Therm. Anal. Calorim. 2004, 76, pp. 335-342, doi: 10.1023 / B: JTAN.0000027833.24442.a0 .
- ^ A b Andrew Streitwieser, Clayton H. Heathcock, Edward M. Kosower: Organic Chemistry. 2nd Edition. Wiley-VCH, Weinheim 1994, ISBN 3-527-29005-2 , p. 1227.
- ↑ Harold Hart, Leslie E. Craine, David J. Hart, Christopher M. Hadad: Organic Chemistry. translated into German by Nicole Kindler. 3. Edition. Wiley-VCH, Weinheim 2007, ISBN 978-3-527-31801-8 , p. 494.
- ↑ Detlef Gerritzen: Economy and ecology in harmony - sustainable chemical production using the example of the Lonza production network in Visp. (PDF; 695 kB), 7th Freiburg Symposium 2005, Sustainable Chemical Production.
- ↑ JB Tarr, J. Arditti: Niacin Biosynthesis in Seedlings of Zea mays. In: Plant Physiol. 1982, 69 (3), pp. 553-556, PMID 16662247 , PMC 426252 (free full text).
- ↑ R. Jacob, M. Swenseid: niacin. In: EE Ziegler, LJ Filer (Ed.): Present Knowledge in Nutrition. 7th edition. ILSI Press, Washington DC 1996, pp. 185-190.
- ↑ CS Fu, ME Swendseid, RA Jacob, RW McKee: Biochemical markers for assessment of niacin status in young men: levels of erythrocyte niacin coenzymes and plasma tryptophan. In: J. Nutr. 1989, 119 (12), pp. 1949-1955, PMID 2621487 , PDF .
- ↑ Hans Konrad Biesalski among others: Nutritional medicine. 4th edition. Thieme Verlag, 2010, ISBN 978-3-13-100294-5 , p. 177.
- ↑ Feed additives in dairy cows ( Memento of the original from April 23, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF; 1.0 MB).
- ↑ The ingestion of nicotinic acid in excessive doses can be harmful to health. Opinion No. 018/2012 of the BfR from February 6, 2012, accessed on November 12, 2016.
- ↑ a b Stefan Schandelmaier, Matthias Briel, Ramon Saccilotto, Kelechi K. Olu, Armon Arpagaus: niacin for primary and secondary prevention of cardiovascular events . In: The Cochrane Database of Systematic Reviews . tape 6 , June 14, 2017, p. CD009744 , doi : 10.1002 / 14651858.CD009744.pub2 , PMID 28616955 .
- ↑ L. Jones Hollis: Abbott down after NIH halts Niaspan study. In: fiercepharma.com of May 26, 2011.
- ↑ NIH stops clinical trial on combination cholesterol treatment. ( Page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. In: fiercepharma.com of May 26, 2011.
- ↑ MSD takes Tredaptive® off the market. In: Deutsche Apotheker Zeitung of January 13, 2013.
Web links
- Swiss Forum For Sport Nutrition: Information sheet Niacin ( Memento from September 7, 2012 in the Internet Archive ) (PDF)