Cytomegalovirus: Difference between revisions
misleading link, different definition of ambulatory Tags: Mobile edit Mobile web edit |
|||
| (760 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Genus of viruses}} |
|||
{{DiseaseDisorder infobox | |
|||
{{Use dmy dates|date=April 2017}} |
|||
Name = Cytomegalovirus | |
|||
{{Virusbox |
|||
ICD10 = {{ICD10|B|25||b|20}} | |
|||
| image = Cytomegalovirus 01.jpg |
|||
| image_alt = Typical "owl eye" intranuclear inclusion indicating CMV infection of a lung pneumocyte |
|||
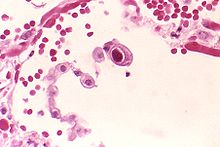
| image_caption = Typical "owl eye" intranuclear [[Inclusion bodies|inclusion]] indicating CMV infection of a lung [[pneumocyte]]<ref>{{cite journal | vauthors = Mattes FM, McLaughlin JE, Emery VC, Clark DA, Griffiths PD | title = Histopathological detection of owl's eye inclusions is still specific for cytomegalovirus in the era of human herpesviruses 6 and 7 | journal = Journal of Clinical Pathology | volume = 53 | issue = 8 | pages = 612–4 | date = August 2000 | pmid = 11002765 | pmc = 1762915 | doi = 10.1136/jcp.53.8.612 }}</ref> |
|||
| taxon = Cytomegalovirus |
|||
| synonyms = |
|||
* ''Human cytomegalovirus group'' |
|||
| synonyms_ref = <ref>{{cite journal | vauthors = Francki RI, Fauquet CM, Knudson DL, Brown | title = Classification and nomenclature of viruses. Fifth Report of the International Committee on Taxonomy of Viruses. | journal = Arch. Virol. | date = 1991 | page = 107 | url = https://ictv.global/ictv/proposals/ICTV%205th%20Report.pdf }}</ref> |
|||
| subdivision_ranks = Species |
|||
| subdivision = See text |
|||
}} |
}} |
||
'''''Cytomegalovirus''''' ('''''CMV''''') (from ''cyto-'' 'cell' via [[Ancient Greek|Greek]] {{lang|grc|κύτος}} {{transliteration|grc|kútos}}- 'container' + {{lang|grc|μέγας}} {{transliteration|grc|mégas}} 'big, megalo-' + -''virus'' via [[Latin]] {{lang|la|vīrus}} 'poison') is a genus of [[virus]]es in the order ''[[Herpesvirales]]'', in the family ''[[Herpesviridae]]'',<ref>{{cite journal | vauthors = Anshu A, Tan D, Chee SP, Mehta JS, Htoon HM | title = Interventions for the management of CMV-associated anterior segment inflammation | journal = The Cochrane Database of Systematic Reviews | volume = 2017 | pages = CD011908 | date = August 2017 | issue = 8 | pmid = 28838031 | pmc = 6483705 | doi = 10.1002/14651858.cd011908.pub2 }}</ref> in the subfamily ''[[Betaherpesvirinae]]''. [[Human]]s and other [[primate]]s serve as natural [[host (biology)|hosts]]. The 11 species in this genus include ''[[human betaherpesvirus 5]]'' (HCMV, human cytomegalovirus, HHV-5), which is the [[species]] that infects humans. Diseases associated with HHV-5 include [[infectious mononucleosis|mononucleosis]] and [[pneumonia]],<ref name=ViralZone>{{cite web|title=Viral Zone|url=http://viralzone.expasy.org/all_by_species/180.html|publisher=ExPASy|access-date=15 June 2015}}</ref><ref name=ICTV>{{cite web |title=Virus Taxonomy: 2022 Release |url=https://ictv.global/taxonomy |publisher=International Committee on Taxonomy of Viruses (ICTV) |date=March 2023 |access-date=16 September 2022}}</ref> and [[congenital]] CMV in infants can lead to deafness and ambulatory problems.<ref name=StatCMV>{{cite web|title=Often overlooked, a common infection during pregnancy kickstarts a conversation about newborn screening|date=5 April 2023 |url=https://www.statnews.com/2023/04/05/cmv-screening-congenital-cytomegalovirus-hearing-loss|publisher=STAT|access-date=5 April 2023}}</ref> |
|||
{{Taxobox | color=violet |
|||
| name = ''Cytomegalovirus'' |
|||
| image = Cytomegalovirus 01.jpg |
|||
| image_caption = CMV infection of a lung [[pneumocyte]]. |
|||
| virus_group = i |
|||
| familia = ''[[Herpesviridae]]'' |
|||
| genus = '''''Cytomegalovirus''''' |
|||
| subdivision_ranks = species |
|||
| subdivision = ''see text'' |
|||
}} |
|||
In the [[medical literature]], most mentions of CMV without further specification refer implicitly to human CMV. Human CMV is the most studied of all cytomegaloviruses.<ref name=Sherris>{{cite book | veditors = Ryan KJ, Ray CG | title = Sherris Medical Microbiology | edition = 4th | pages = 556; 566–9 | publisher = McGraw Hill | year = 2004 | isbn = 978-0-8385-8529-0 }}</ref> |
|||
'''''Cytomegalovirus''''' (CMV), is a genus of [[Herpes]] [[virus]]es; in humans the species is known as '''Human herpesvirus 5''' (HHV-5). It belongs to the ''[[Betaherpesvirinae]]'' subfamily of ''[[Herpesviridae]]''. The name means "very big cell virus". |
|||
[[MX2|MX2/MXB]] was identified as a restriction factor for herpesviruses, which acts at a very early stage of the replication cycle and MX2/MXB restriction of herpesvirus requires GTPase activity.<ref name="Staeheli_2018">{{cite journal | vauthors = Staeheli P, Haller O | title = Human MX2/MxB: a Potent Interferon-Induced Postentry Inhibitor of Herpesviruses and HIV-1 | journal = Journal of Virology | volume = 92 | issue = 24 | pages = | date = December 2018 | pmid = 30258007 | pmc = 6258936 | doi = 10.1128/JVI.00709-18 }}</ref> |
|||
CMV especially attacks [[salivary gland]]s. CMV infection can also be life threatening for patients who are [[immunocompromised]] (e.g. patients with [[HIV]], [[organ transplant]] recipients, or neonates). CMV viruses are found in many [[mammal]] species, but CMV [[species]] isolated from animals differ from human CMV in terms of genomic structure, and have not been reported to cause human disease. |
|||
== |
== Taxonomy == |
||
Within the ''[[Herpesviridae]]'', CMV belongs to the ''[[Betaherpesvirinae]]'' subfamily, which also includes the genera ''[[Muromegalovirus]]'' and ''[[Roseolovirus]]'' ([[human herpesvirus 6]] and ''[[human betaherpesvirus 7]]'').<ref name="isbn0-521-82714-0">{{cite book | vauthors = Koichi Y, Arvin AM, Campadelli-Fiume G, Mocarski E, Patrick M, Roizman B, Whitley R |title=Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis |publisher=Cambridge University Press |location=Cambridge, UK |year=2007 |isbn=978-0-521-82714-0 }}</ref> It is also related to other herpesviruses within the ''[[Alphaherpesvirinae]]'' subfamily, which includes [[herpes simplex virus]]es 1 and 2 and [[varicella-zoster virus]], and the ''[[Gammaherpesvirinae]]'' subfamily, which includes [[Epstein–Barr virus]] and [[Kaposi's sarcoma-associated herpesvirus]].<ref name="Sherris" /> |
|||
* ''[[Cercopithecine herpesvirus 5]]'' (CeHV-5) - African green monkey cytomegalovirus |
|||
* ''[[Cercopithecine herpesvirus 8]]'' (CeHV-8) - Rhesus monkey cytomegalovirus |
|||
* ''[[Human herpesvirus 5]]'' (HHV-5) - Human cytomegalovirus |
|||
* ''[[Pongine herpesvirus 4]]'' (PoHV-4) |
|||
Several species of ''Cytomegalovirus'' have been identified and classified for different [[mammal]]s.<ref name="isbn0-521-82714-0"/> The most studied is ''Human cytomegalovirus'' (HCMV), which is also known as ''Human betaherpesvirus 5'' (HHV-5). Other primate CMV species include ''Chimpanzee cytomegalovirus'' (CCMV) that infects [[Common chimpanzee|chimpanzee]]s and [[orangutan]]s, and ''Simian cytomegalovirus'' (SCCMV) and ''Rhesus cytomegalovirus'' (RhCMV) that infect [[macaque]]s; CCMV is known as both ''Panine beta herpesvirus 2'' (PaHV-2) and ''Pongine betaherpesvirus 4'' (PoHV-4).<ref>{{cite web|url=https://www.uniprot.org/taxonomy/188763|title=Panine betaherpesvirus 2 (Chimpanzee cytomegalovirus)|website=www.uniprot.org|access-date=13 March 2019}}</ref> SCCMV is called ''cercopithecine betaherpesvirus 5'' (CeHV-5)<ref>{{cite web|url=https://www.uniprot.org/taxonomy/50292|title=Simian cytomegalovirus (strain Colburn)|website=www.uniprot.org|access-date=13 March 2019}}</ref> and RhCMV, ''Cercopithecine betaherpesvirus 8'' (CeHV-8).<ref>{{cite web|url=https://www.uniprot.org/taxonomy/47929|title=Macacine betaherpesvirus 3 (Rhesus cytomegalovirus)|website=www.uniprot.org|access-date=13 March 2019}}</ref> A further two viruses found in the [[night monkey]] are tentatively placed in the genus ''Cytomegalovirus'', and are called ''Herpesvirus aotus 1'' and ''Herpesvirus aotus 3''. Rodents also have viruses previously called cytomegaloviruses that are now reclassified under the genus ''[[Muromegalovirus]]''; this genus contains ''Mouse cytomegalovirus'' (MCMV) is also known as ''Murid betaherpesvirus 1'' (MuHV-1) and the closely related ''Murid betaherpesvirus 2'' (MuHV-2) that is found in [[rat]]s.<ref>{{cite web|url=http://www.ncbi.nlm.nih.gov/nuccore/NC_004065.1|title=Murid herpesvirus 1, complete genome|date=13 August 2018|access-date=13 March 2019|via=NCBI Nucleotide}}</ref> |
|||
Tentative species: |
|||
* ''[[Aotine herpesvirus 1]]'' (AoHV-1) - Herpesvirus aotus 1 |
|||
* ''[[Aotine herpesvirus 3]]'' (AoHV-3) - Herpesvirus aotus 3 |
|||
== |
=== Species === |
||
The following 11 species are assigned to the genus in ICTV 2022:<ref name=ICTV /> |
|||
Cytomegalovirus, or CMV, is found universally throughout all geographic locations and socioeconomic groups, and infects between 50% and 85% of adults in the United States by 40 years of age. This can be seen by the presence of [[antibodies]] in much of the general population, indicating they had been infected at one point. CMV is also the [[virus]] most frequently transmitted to a developing child before birth. CMV [[infection]] is more widespread in developing countries and in areas of lower socioeconomic conditions. It causes the most birth defects in industrialized countries out of all the herpes viruses. For most healthy persons who acquire CMV after birth there are few symptoms. Some persons with symptoms experience [[infectious mononucleosis]], with prolonged [[fever]], and a mild [[hepatitis]]. A very sore throat is also common. Once a person becomes infected, the virus latently persists in the body for the person's life and can exhaust the immune system at old age, increasing risk of mortality from other diseases. Recurrent disease rarely occurs unless the person's [[immune system]] is suppressed due to therapeutic drugs or disease. Therefore, for the vast majority of people, CMV infection is not a serious problem. |
|||
{{div col|colwidth=20em}} |
|||
* ''[[Aotine betaherpesvirus 1|Cytomegalovirus aotinebeta1]]'' |
|||
* ''[[Cebine betaherpesvirus 1|Cytomegalovirus cebinebeta1]]'' |
|||
* ''[[Cercopithecine betaherpesvirus 5|Cytomegalovirus cercopithecinebeta5]]'' |
|||
* ''[[Human betaherpesvirus 5|Cytomegalovirus humanbeta5]]'' |
|||
* ''[[Macacine betaherpesvirus 3|Cytomegalovirus macacinebeta3]]'' |
|||
* ''[[Macacine betaherpesvirus 8|Cytomegalovirus macacinebeta8]]'' |
|||
* ''[[Mandrilline betaherpesvirus 1|Cytomegalovirus mandrillinebeta1]]'' |
|||
* ''[[Panine betaherpesvirus 2|Cytomegalovirus paninebeta2]]'' |
|||
* ''[[Papiine betaherpesvirus 3|Cytomegalovirus papiinebeta3]]'' |
|||
* ''[[Papiine betaherpesvirus 4|Cytomegalovirus papiinebeta4]]'' |
|||
* ''[[Saimiriine betaherpesvirus 4|Cytomegalovirus saimiriinebeta4]]'' |
|||
{{div col end}} |
|||
== Structure == |
|||
However, CMV infection is important to certain high-risk groups. Major areas of risk of infection are: |
|||
[[File:CMVschema.svg|thumb|right|Schematic of a ''Cytomegalovirus'']] |
|||
# To the fetus during [[pregnancy]] |
|||
Viruses in ''Cytomegalovirus'' are enveloped, with icosahedral, spherical to pleomorphic, and round geometries, and T=16 symmetry. The diameter is around 150–200 nm. Genomes are linear and nonsegmented, around 200 kb in length.<ref name=ViralZone /> |
|||
# To people who work with children |
|||
# To the immunocompromised person, such as [[organ transplant]] recipients, persons with [[leukemia]], and persons infected with human immunodeficiency virus ([[HIV]]). It is an [[AIDS]] defining infection, indicating that the [[T-cell]] count has dropped to low levels. |
|||
# To newborn babies |
|||
{| class="wikitable sortable" style="text-align:center" |
|||
[[Lytic cycle|Lytically replicating]] virus disrupts the [[cytoskeleton]], causing massive cell enlargement, which is the source of the virus' name. |
|||
|- |
|||
! Genus !! Structure || Symmetry !! Capsid !! Genomic arrangement !! Genomic segmentation |
|||
|- |
|||
|''Cytomegalovirus''||Spherical pleomorphic||T=16||Enveloped||Linear||Monopartite |
|||
|} |
|||
== Genome == |
|||
==Characteristics of the virus== |
|||
[[File:HCMV genome.png|thumb|Class E genome of HCMV. The unique long and unique short regions are indicated as UL and US. Repeat regions are indicated as a, b and c sequences, where primes designate inverted orientations. Sequences ''ab'' and ''b′a′'' correspond to the terminal/internal repeat long (TRL/IRL); sequences ''a′c′'' and ''ca'' correspond to the internal/terminal repeat short (IRS/TRS). '''Top''': typical genome arrangement of wild-type strains; '''bottom''': genome arrangement of strain AD169, a laboratory-adapted strain. Genome rearrangements that have occurred during extensive passaging are indicated in red between the wild-type and laboratory-adapted configurations.<ref name=":0" />|alt=|400px]] |
|||
CMV is a member of the herpesvirus group, which includes [[herpes simplex virus]] types 1 and 2, [[varicella-zoster virus]] (which causes [[chickenpox]] and [[shingles]]), and [[Epstein-Barr]] virus (which, together with CMV, are the main causes for [[infectious mononucleosis]]). These viruses share a characteristic ability to remain latent within the body over a long period. |
|||
Herpesviruses have some of the largest genomes among human viruses, often encoding hundreds of proteins. For instance, the double‑stranded DNA (dsDNA) [[genome]] of wild-type HCMV strains has a size of around 235 kb and encodes at least 208 proteins. It is thus longer than all other human herpesviruses and one of the longest genomes of all human viruses in general. It has the characteristic herpesvirus class E genome architecture, consisting of two unique regions (unique long UL and unique short US), both flanked by a pair of inverted repeats (terminal/internal repeat long TRL/IRL and internal/terminal repeat short IRS/TRS). Both sets of repeats share a region of a few hundred bps, the so-called "a sequence"; the other regions of the repeats are sometimes referred to as "b sequence" and "c sequence".<ref name=":0">{{cite journal | vauthors = Sijmons S, Van Ranst M, Maes P | title = Genomic and functional characteristics of human cytomegalovirus revealed by next-generation sequencing | journal = Viruses | volume = 6 | issue = 3 | pages = 1049–1072 | date = March 2014 | pmid = 24603756 | pmc = 3970138 | doi = 10.3390/v6031049 | doi-access = free }}</ref> |
|||
Initial CMV infection, which may have few symptoms, is always followed by a prolonged, inapparent infection during which the virus resides in cells without causing detectable damage or clinical illness. Severe impairment of the body's immune system by medication or disease (see below) may reactivate the virus from the latent or dormant state. |
|||
== {{anchor|Lifecycle}}Life cycle == |
|||
Infectious CMV may be shed in the bodily fluids of any previously infected person, and thus may be found in [[urine]], [[saliva]], [[blood]], [[tears]], [[semen]], and [[breast milk]]. The shedding of virus may take place intermittently, without any detectable signs, and without causing symptoms. |
|||
Viral replication is nuclear and [[Lysogenic cycle|lysogenic.]] Entry into the host cell is achieved by attachment of the viral [[glycoproteins]] to host receptors, which mediates [[endocytosis]]. [[DNA replication|Replication]] follows the dsDNA bidirectional replication model. [[Transcription (genetics)#Elongation|DNA templated transcription]], with some [[alternative splicing]] mechanism is the method of transcription. [[Translation (biology)|Translation]] takes place by [[leaky scanning]]. The virus exits the host cell by [http://viralzone.expasy.org/all_by_species/1952.html nuclear egress], and [[Viral shedding#Via budding|budding.]] Humans and monkeys serve as the natural hosts. Transmission routes are dependent on coming into contact with bodily fluids (such as saliva, urine, and genital secretions) from an infected individual.<ref name=ViralZone /><ref>{{cite journal | vauthors = Cannon MJ, Hyde TB, Schmid DS | title = Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection | journal = Reviews in Medical Virology | volume = 21 | issue = 4 | pages = 240–255 | date = July 2011 | pmid = 21674676 | pmc = 4494736 | doi = 10.1002/rmv.695 }}</ref> |
|||
{| class="wikitable sortable" style="text-align:center" |
|||
Microscopically, CMV can be demonstrated by intranuclear [[inclusion]] bodies, which show that the [[virus]] replicates in the nucleus rather than the cytosol. These inclusion bodies stain dark pink on an [[H&E stain]], and are also called "Owl's Eye" inclusion bodies. |
|||
|- |
|||
! Genus !! Host details !! Tissue tropism !! Entry details !! Release details !! Replication site !! Assembly site !! Transmission |
|||
|- |
|||
|''Cytomegalovirus''||humans; monkeys||Epithelial mucosa||Glycoproteins||Budding||Nucleus||Nucleus||Urine; saliva; congenital |
|||
|} |
|||
All [[human herpesviruses|herpesviruses]] share a characteristic ability to remain [[Virus latency|latent]] within the body over long periods. Although they may be found throughout the body, CMV infections are frequently associated with the [[salivary gland]]s in humans and other [[mammal]]s.<ref name="isbn0-521-82714-0"/> |
|||
As a result of efforts to create an attenuated-virus vaccine, there currently exist two general classes of CMV. ''Clinical isolates'' comprise those viruses obtained from patients and represent the wild-type viral genome, while ''laboratory strains'' have been cultured extensively in the lab setting and typically contain numerous accumulated mutations. Most notably, the laboratory strain AD169 appears to lack a 15kb region of the 200kb genome that is present in clinical isolates. This region contains 19 [[open reading frame]]s whose functions have yet to be elucidated. AD169 is also unique in that it is unable to enter latency and nearly always assumes lytic growth upon infection. |
|||
==Genetic engineering== |
|||
==Transmission and prevention== |
|||
The CMV promoter is commonly included in [[Vector (molecular biology)|vectors]] used in [[genetic engineering]] work conducted in [[mammal]]ian cells, as it is a strong [[promoter (genetics)|promoter]] and drives constitutive expression of genes under its control.<ref>{{cite web | first = Kendall | last = Morgan | name-list-style = vanc | work = Addgene Blog | date = 3 April 2014 | url = http://blog.addgene.org/plasmids-101-the-promoter-region | title = Plasmids 101: The Promoter Region – Let's Go! }}</ref> |
|||
Transmission of CMV occurs from person to person. 58.9% of individuals aged 6 and over are infected with CMV; this number rises to 90.8% of individuals aged 80 and over.<ref>{{cite journal | author=Staras SAS, Dollard SC, Radford KW, ''et al.'' | title=Seroprevalence of cytomegalovirus infection in the United States, 1988–1994 | year=2006 | journal=Clin Infect Dis | volume=43 | pages=1143–51 }}</ref> It is debatable whether any measures to prevent infection should be taken at all when dealing with someone with CMV, as infection is almost universal in all countries. |
|||
== History == |
|||
Infection requires close, intimate contact with a person excreting the virus in their saliva, urine, or other bodily fluids. CMV can be [[Sexually transmitted disease|sexually transmitted]] and can also be transmitted via [[Breastfeeding|breast milk]], transplanted organs, and rarely from [[blood transfusion]]s. |
|||
''Cytomegalovirus'' was first observed by German pathologist [[Hugo Ribbert]] in 1881 when he noticed enlarged cells with enlarged nuclei present in the cells of an infant.<ref>{{Cite book | veditors = Reddehase MJ, Lemmermann N | pages = xxiv |chapter=Preface |title=Cytomegaloviruses: Molecular Biology and Immunology |publisher=Horizon Scientific Press |year=2006 |isbn=9781904455028 }}</ref> Years later, between 1956 and 1957, [[Thomas Huckle Weller]] together with Smith and Rowe independently isolated the virus, known thereafter as "cytomegalovirus".<ref>{{cite journal | vauthors = Craig JM, Macauley JC, Weller TH, Wirth P | title = Isolation of intranuclear inclusion producing agents from infants with illnesses resembling cytomegalic inclusion disease | journal = Proceedings of the Society for Experimental Biology and Medicine | volume = 94 | issue = 1 | pages = 4–12 | date = January 1957 | pmid = 13400856 | doi = 10.3181/00379727-94-22841 | s2cid = 29263626 }}</ref> In 1990, the first draft of human cytomegalovirus genome was published,<ref>{{cite book | vauthors = Chee MS, Bankier AT, Beck S, Bohni R, Brown CM, Cerny R, Horsnell T, Hutchison CA, Kouzarides T, Martignetti JA | chapter = Analysis of the Protein-Coding Content of the Sequence of Human Cytomegalovirus Strain AD169 | title = Cytomegaloviruses | display-authors = 6 | volume = 154 | pages = 125–69 | date = 1990 | pmid = 2161319 | doi = 10.1007/978-3-642-74980-3_6 | publisher = Springer Berlin Heidelberg | isbn = 978-3-642-74982-7 | series = Current Topics in Microbiology and Immunology }}</ref> the biggest contiguous genome sequenced at that time.<ref>{{cite journal | vauthors = Martí-Carreras J, Maes P | title = Human cytomegalovirus genomics and transcriptomics through the lens of next-generation sequencing: revision and future challenges | journal = Virus Genes | volume = 55 | issue = 2 | pages = 138–164 | date = April 2019 | pmid = 30604286 | pmc = 6458973 | doi = 10.1007/s11262-018-1627-3 }}</ref> |
|||
== See also == |
|||
Although the virus is not highly contagious, it has been shown to spread in households and among young children in day care centers. Transmission of the virus is often preventable because it is most often transmitted through infected bodily fluids that come in contact with hands and then are absorbed through the nose or mouth of a susceptible person. Therefore, care should be taken when handling children and items like diapers. Simple hand washing with soap and water is effective in removing the virus from the hands. |
|||
{{Portal|Viruses}} |
|||
* [[CMV polyradiculomyelopathy]] |
|||
* [[Human betaherpesvirus 5|Human cytomegalovirus]] |
|||
== References == |
|||
CMV infection without symptoms is common in infants and young children; as a result, it is common to not exclude from school or an institution a child known to be infected. Similarly, hospitalized patients are not typically separated or isolated. |
|||
{{Reflist}} |
|||
== External links == |
|||
==Specific situations== |
|||
{{Commons category}} |
|||
===Pregnancy=== |
|||
{{Wikispecies}} |
|||
The incidence of primary <!--(or first)--> CMV infection in pregnant women in the United States varies from 1% to 3%. Healthy pregnant women are not at special risk for disease from CMV infection. When infected with CMV, most women have no symptoms and very few have a disease resembling mononucleosis. It is their developing fetuses that may be at risk for congenital CMV disease. CMV remains the most important cause of congenital <!--(meaning from birth)--> viral infection in the United States. For infants who are infected by their mothers before birth, two potential problems exist: |
|||
* [http://ictvonline.org/virusTaxonomy.asp ICTV] |
|||
*Generalized infection may occur in the infant, and symptoms may range from moderate enlargement of the liver and spleen ([[Hepatosplenomegaly]]) (with [[jaundice]]) to fatal illness. With supportive treatment most infants with CMV disease usually survive. However, from 80% to 90% will have complications within the first few years of life that may include hearing loss, vision impairment, and varying degrees of mental retardation. |
|||
*Another 5% to 10% of infants who are infected but without symptoms at birth will subsequently have varying degrees of hearing and mental or coordination problems. |
|||
{{Medical resources |
|||
However, these risks appear to be almost exclusively associated with women who previously have not been infected with CMV and who are having their first infection with the virus during pregnancy. Even in this case, two-thirds of the infants will not become infected, and only 10% to 15% of the remaining third will have symptoms at the time of birth. There appears to be little risk of CMV-related complications for women who have been infected at least 6 months prior to conception. For this group, which makes up 50% to 80% of the women of child-bearing age, the rate of newborn CMV infection is 1%, and these infants appear to have no significant illness or abnormalities.{{Fact|date=February 2007}} |
|||
| ICD10 = {{ICD10|B|25||b|25}} |
|||
| ICD9 = {{ICD9|078.5}} |
|||
| MedlinePlus = 000568 |
|||
| MeshID = D003586 |
|||
}} |
|||
{{Herpesvirales}} |
|||
The virus can also be transmitted to the infant at delivery from contact with genital secretions or later in infancy through breast milk. However, these infections usually result in little or no clinical illness in the infant. |
|||
{{Baltimore classification}} |
|||
{{Viral diseases}} |
|||
To summarize, during a pregnancy when a woman who has never had CMV infection becomes infected with CMV, there is a potential risk that after birth the infant may have CMV-related complications, the most common of which are associated with hearing loss, visual impairment, or diminished mental and motor capabilities. On the other hand, infants and children who acquire CMV after birth have few, if any, symptoms or complications. |
|||
{{Diseases of maternal transmission}} |
|||
{{Taxonbar|from=Q6946}} |
|||
Recommendations for pregnant women with regard to CMV infection: |
|||
{{Authority control}} |
|||
*Throughout the pregnancy, practice good personal hygiene, especially handwashing with soap and water, after contact with diapers or oral secretions (particularly with a child who is in day care). |
|||
*Women who develop a mononucleosis-like illness during pregnancy should be evaluated for CMV infection and counseled about the possible risks to the unborn child. |
|||
*Laboratory testing for antibody to CMV can be performed to determine if a women has already had CMV infection. |
|||
*Recovery of CMV from the cervix or urine of women at or before the time of delivery does not warrant a cesarean section. |
|||
*The demonstrated benefits of breast-feeding outweigh the minimal risk of acquiring CMV from the breast-feeding mother. |
|||
*There is no need to either screen for CMV or exclude CMV-excreting children from schools or institutions because the virus is frequently found in many healthy children and adults. |
|||
===Childcare=== |
|||
Most *Pregnant women working with infants and children should be informed of the risk of acquiring CMV infection and the possible effects on the unborn child. |
|||
*Routine laboratory testing for CMV antibody in female workers is not recommended, but can be performed to determine their immune status |
|||
===Immunocompromised patients=== |
|||
Primary <!--(or the initial)--> CMV infection in the immunocompromised patient can cause serious disease. However, the more common problem is the reactivation of the latent virus. |
|||
In patients with a depressed immune system, CMV-related disease may be much more aggressive. CMV hepatitis may cause fulminant [[liver failure]]. Specific disease entities recognised in those people are [[cytomegalovirus retinitis]] (inflammation of the [[retina]], characterised by a "pizza pie appearance" on [[ophthalmoscopy]]) and [[cytomegalovirus colitis]] (inflammation of the [[colon (anatomy)|large bowel]]). |
|||
Infection with CMV is a major cause of disease and death in immunocompromised patients, including organ transplant recipients, patients undergoing hemo[[dialysis]], patients with [[cancer]], patients receiving [[immunosuppressive]] drugs, and [[HIV]]-infected patients. Because of this risk, exposing immunosuppressed patients to outside sources of CMV should be minimized. Whenever possible, patients without CMV infection should be given organs and/or blood products that are free of the virus. |
|||
Patients without CMV infection who are given organ transplants from CMV-infected donors should be given prophlyactic treatment with [[valganciclovir]] (ideally) or [[ganciclovir]] and require regular serological monitoring to detect a rising CMV titre, which should be treated early to prevent a potentially life-threatening infection becoming established. |
|||
==Diagnosis of infection== |
|||
Most infections with CMV are not diagnosed because the virus usually produces few, if any, symptoms and tends to reactivate intermittently without symptoms. However, persons who have been infected with CMV develop [[antibodies]] to the virus, and these antibodies persist in the body for the lifetime of that individual. A number of laboratory tests that detect these antibodies to CMV have been developed to determine if infection has occurred and are widely available from commercial laboratories. In addition, the virus can be cultured from specimens obtained from urine, throat swabs, bronchial lavages and tissue samples to detect active infection. Both qualitative and quantitative [[PCR]] testing for CMV are available as well, allowing physicians to monitor the [[viral load]] of CMV-infected patients. |
|||
CMV should be suspected if a patient: |
|||
*Has symptoms of infectious mononucleosis but has negative test results for mononucleosis and Epstein Barr virus, or, |
|||
*Shows signs of hepatitis, but has negative test results for hepatitis A, B, and C. |
|||
For best diagnostic results, laboratory tests for CMV antibody should be performed by using paired serum samples. One blood sample should be taken upon suspicion of CMV, and another one taken within 2 weeks. A virus culture can be performed at any time the patient is symptomatic. |
|||
Laboratory testing for antibody to CMV can be performed to determine if a woman has already had CMV infection. However, routine laboratory testing of all pregnant women is costly and the need for testing should therefore be evaluated on a case-by-case basis. |
|||
===Serologic testing=== |
|||
The enzyme-linked immunosorbent assay (or [[ELISA]]) is the most commonly available serologic test for measuring antibody to CMV. The result can be used to determine if acute infection, prior infection, or passively acquired maternal antibody in an infant is present. Other tests include various [[fluorescence assay]]s, [[indirect hemagglutination]], polymerase chain reaction ([[PCR]]) and [[latex agglutination]]. |
|||
An ELISA technique for CMV-specific IgM is available, but may give false-positive results unless steps are taken to remove rheumatoid factor or most of the IgG antibody before the serum sample is tested. Because CMV-specific IgM may be produced in low levels in reactivated CMV infection, its presence is not always indicative of primary infection. Only virus recovered from a target organ, such as the lung, provides unequivocal evidence that the current illness is caused by acquired CMV infection. If serologic tests detect a positive or high titer of IgG, this result should not automatically be interpreted to mean that active CMV infection is present. However, if antibody tests of paired serum samples show a fourfold rise in IgG antibody and a significant level of IgM antibody, meaning equal to at least 30% of the IgG value, or virus is cultured from a urine or throat specimen, the findings indicate that an active CMV infection is present. |
|||
=== Relevance to blood donors === |
|||
Although the risks discussed above are generally low, CMV assays are part of the standard screening for non-directed [[blood donation]] (donations not specified for a particular patient) in the U.S. CMV-negative donations are then earmarked for transfusion to infants or immunocompromised patients. Some blood donation[http://www.unitedbloodservices.org/faqs.html] centers may maintain lists of donors whose blood is CMV negative due to special demands. |
|||
==Treatment== |
|||
No treatment is generally necessary for CMV infection in the healthy individual since the majority of infections resolve on their own. Antiviral drug therapy is now being evaluated in infants. |
|||
[[Ganciclovir]] treatment is used for patients with depressed immunity who have either sight-related or life-threatening illnesses. [[Valganciclovir]] (marketed as Valcyte) is an antiviral drug that is also effective and is given orally. [[Foscarnet]] or cidofovir can be given in patients with CMV resistant to ganciclovir, though foscarnet is not as well tolerated as ganciclovir. |
|||
Vaccines are still in the research and development stage. |
|||
==Open Questions== |
|||
1) Do people infected with CMV suffer chronic deterioration in their cognitive abilities (memory, I.Q., ...)? |
|||
2) Is there any scientific research showing correlation between lower I.Q. and CMV infection? |
|||
3) What are the conditions under which people develop CMV mononucleosis? |
|||
==Support group== |
|||
For families in UK with a child with congenital CMV damage, support is available from the Congenital CMv Association, 128 Northfileds Lane, Brixham, Devon TQ5 8RH. Phone 01803 856496. |
|||
==External links== |
|||
*[http://www.gfmer.ch/genetic_diseases_v2/gendis_detail_list.php?cat3=349 Cytomegalovirus infection, congenital - Geneva Foundation for Medical Education and Research] |
|||
*[http://www.cdc.gov/cmv CDC information on cytomegalovirus] |
|||
*[http://www.transfusionguidelines.org.uk/index.asp?Publication=DL&Section=12&pageid=807 CMV Seronegative vs. Leucodepleted blood components for at risk recipients.] |
|||
*[http://www.aabb.org/Content/About_Blood/Facts_About_Blood_and_Blood_Banking/fabloodtrans.htm#6 Cytomegalovirus (CMV)] |
|||
==References== |
|||
#Fletcher JM, Vukmanovic-Stejic M, Dunne PJ, Birch KE, Cook JE, Jackson SE, Salmon M, Rustin MH, and Akbar AN. "Cytomegalovirus-Specific CD4+ T Cells in Healthy Carriers Are Continuously Driven To Replicative Exhaustion." J Immunol. Vol. 175, No. 12, pp. 8218-25 PMID 16339561 |
|||
[[Category:Viral diseases]] |
[[Category:Viral diseases]] |
||
[[Category: |
[[Category:Betaherpesvirinae]] |
||
[[Category:Virus genera]] |
|||
[[de:Zytomegalievirus]] |
|||
[[es:Citomegalovirus]] |
|||
[[fr:Cytomégalovirus]] |
|||
[[it:Citomegalovirus]] |
|||
[[nl:Cytomegalovirus]] |
|||
[[ja:サイトメガロウイルス]] |
|||
[[no:Cytomegalovirus]] |
|||
[[pl:Cytomegalowirus]] |
|||
[[fi:Sytomegalovirus]] |
|||
[[sv:CMV]] |
|||
Latest revision as of 03:25, 11 February 2024
| Cytomegalovirus | |
|---|---|
| |
| Typical "owl eye" intranuclear inclusion indicating CMV infection of a lung pneumocyte[1] | |
| Virus classification | |
| (unranked): | Virus |
| Realm: | Duplodnaviria |
| Kingdom: | Heunggongvirae |
| Phylum: | Peploviricota |
| Class: | Herviviricetes |
| Order: | Herpesvirales |
| Family: | Orthoherpesviridae |
| Subfamily: | Betaherpesvirinae |
| Genus: | Cytomegalovirus |
| Species | |
|
See text | |
| Synonyms[2] | |
| |
Cytomegalovirus (CMV) (from cyto- 'cell' via Greek κύτος kútos- 'container' + μέγας mégas 'big, megalo-' + -virus via Latin vīrus 'poison') is a genus of viruses in the order Herpesvirales, in the family Herpesviridae,[3] in the subfamily Betaherpesvirinae. Humans and other primates serve as natural hosts. The 11 species in this genus include human betaherpesvirus 5 (HCMV, human cytomegalovirus, HHV-5), which is the species that infects humans. Diseases associated with HHV-5 include mononucleosis and pneumonia,[4][5] and congenital CMV in infants can lead to deafness and ambulatory problems.[6]
In the medical literature, most mentions of CMV without further specification refer implicitly to human CMV. Human CMV is the most studied of all cytomegaloviruses.[7]
MX2/MXB was identified as a restriction factor for herpesviruses, which acts at a very early stage of the replication cycle and MX2/MXB restriction of herpesvirus requires GTPase activity.[8]
Taxonomy[edit]
Within the Herpesviridae, CMV belongs to the Betaherpesvirinae subfamily, which also includes the genera Muromegalovirus and Roseolovirus (human herpesvirus 6 and human betaherpesvirus 7).[9] It is also related to other herpesviruses within the Alphaherpesvirinae subfamily, which includes herpes simplex viruses 1 and 2 and varicella-zoster virus, and the Gammaherpesvirinae subfamily, which includes Epstein–Barr virus and Kaposi's sarcoma-associated herpesvirus.[7]
Several species of Cytomegalovirus have been identified and classified for different mammals.[9] The most studied is Human cytomegalovirus (HCMV), which is also known as Human betaherpesvirus 5 (HHV-5). Other primate CMV species include Chimpanzee cytomegalovirus (CCMV) that infects chimpanzees and orangutans, and Simian cytomegalovirus (SCCMV) and Rhesus cytomegalovirus (RhCMV) that infect macaques; CCMV is known as both Panine beta herpesvirus 2 (PaHV-2) and Pongine betaherpesvirus 4 (PoHV-4).[10] SCCMV is called cercopithecine betaherpesvirus 5 (CeHV-5)[11] and RhCMV, Cercopithecine betaherpesvirus 8 (CeHV-8).[12] A further two viruses found in the night monkey are tentatively placed in the genus Cytomegalovirus, and are called Herpesvirus aotus 1 and Herpesvirus aotus 3. Rodents also have viruses previously called cytomegaloviruses that are now reclassified under the genus Muromegalovirus; this genus contains Mouse cytomegalovirus (MCMV) is also known as Murid betaherpesvirus 1 (MuHV-1) and the closely related Murid betaherpesvirus 2 (MuHV-2) that is found in rats.[13]
Species[edit]
The following 11 species are assigned to the genus in ICTV 2022:[5]
- Cytomegalovirus aotinebeta1
- Cytomegalovirus cebinebeta1
- Cytomegalovirus cercopithecinebeta5
- Cytomegalovirus humanbeta5
- Cytomegalovirus macacinebeta3
- Cytomegalovirus macacinebeta8
- Cytomegalovirus mandrillinebeta1
- Cytomegalovirus paninebeta2
- Cytomegalovirus papiinebeta3
- Cytomegalovirus papiinebeta4
- Cytomegalovirus saimiriinebeta4
Structure[edit]

Viruses in Cytomegalovirus are enveloped, with icosahedral, spherical to pleomorphic, and round geometries, and T=16 symmetry. The diameter is around 150–200 nm. Genomes are linear and nonsegmented, around 200 kb in length.[4]
| Genus | Structure | Symmetry | Capsid | Genomic arrangement | Genomic segmentation |
|---|---|---|---|---|---|
| Cytomegalovirus | Spherical pleomorphic | T=16 | Enveloped | Linear | Monopartite |
Genome[edit]

Herpesviruses have some of the largest genomes among human viruses, often encoding hundreds of proteins. For instance, the double‑stranded DNA (dsDNA) genome of wild-type HCMV strains has a size of around 235 kb and encodes at least 208 proteins. It is thus longer than all other human herpesviruses and one of the longest genomes of all human viruses in general. It has the characteristic herpesvirus class E genome architecture, consisting of two unique regions (unique long UL and unique short US), both flanked by a pair of inverted repeats (terminal/internal repeat long TRL/IRL and internal/terminal repeat short IRS/TRS). Both sets of repeats share a region of a few hundred bps, the so-called "a sequence"; the other regions of the repeats are sometimes referred to as "b sequence" and "c sequence".[14]
Life cycle[edit]
Viral replication is nuclear and lysogenic. Entry into the host cell is achieved by attachment of the viral glycoproteins to host receptors, which mediates endocytosis. Replication follows the dsDNA bidirectional replication model. DNA templated transcription, with some alternative splicing mechanism is the method of transcription. Translation takes place by leaky scanning. The virus exits the host cell by nuclear egress, and budding. Humans and monkeys serve as the natural hosts. Transmission routes are dependent on coming into contact with bodily fluids (such as saliva, urine, and genital secretions) from an infected individual.[4][15]
| Genus | Host details | Tissue tropism | Entry details | Release details | Replication site | Assembly site | Transmission |
|---|---|---|---|---|---|---|---|
| Cytomegalovirus | humans; monkeys | Epithelial mucosa | Glycoproteins | Budding | Nucleus | Nucleus | Urine; saliva; congenital |
All herpesviruses share a characteristic ability to remain latent within the body over long periods. Although they may be found throughout the body, CMV infections are frequently associated with the salivary glands in humans and other mammals.[9]
Genetic engineering[edit]
The CMV promoter is commonly included in vectors used in genetic engineering work conducted in mammalian cells, as it is a strong promoter and drives constitutive expression of genes under its control.[16]
History[edit]
Cytomegalovirus was first observed by German pathologist Hugo Ribbert in 1881 when he noticed enlarged cells with enlarged nuclei present in the cells of an infant.[17] Years later, between 1956 and 1957, Thomas Huckle Weller together with Smith and Rowe independently isolated the virus, known thereafter as "cytomegalovirus".[18] In 1990, the first draft of human cytomegalovirus genome was published,[19] the biggest contiguous genome sequenced at that time.[20]
See also[edit]
References[edit]
- ^ Mattes FM, McLaughlin JE, Emery VC, Clark DA, Griffiths PD (August 2000). "Histopathological detection of owl's eye inclusions is still specific for cytomegalovirus in the era of human herpesviruses 6 and 7". Journal of Clinical Pathology. 53 (8): 612–4. doi:10.1136/jcp.53.8.612. PMC 1762915. PMID 11002765.
- ^ Francki RI, Fauquet CM, Knudson DL, Brown (1991). "Classification and nomenclature of viruses. Fifth Report of the International Committee on Taxonomy of Viruses" (PDF). Arch. Virol.: 107.
- ^ Anshu A, Tan D, Chee SP, Mehta JS, Htoon HM (August 2017). "Interventions for the management of CMV-associated anterior segment inflammation". The Cochrane Database of Systematic Reviews. 2017 (8): CD011908. doi:10.1002/14651858.cd011908.pub2. PMC 6483705. PMID 28838031.
- ^ a b c "Viral Zone". ExPASy. Retrieved 15 June 2015.
- ^ a b "Virus Taxonomy: 2022 Release". International Committee on Taxonomy of Viruses (ICTV). March 2023. Retrieved 16 September 2022.
- ^ "Often overlooked, a common infection during pregnancy kickstarts a conversation about newborn screening". STAT. 5 April 2023. Retrieved 5 April 2023.
- ^ a b Ryan KJ, Ray CG, eds. (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. pp. 556, 566–9. ISBN 978-0-8385-8529-0.
- ^ Staeheli P, Haller O (December 2018). "Human MX2/MxB: a Potent Interferon-Induced Postentry Inhibitor of Herpesviruses and HIV-1". Journal of Virology. 92 (24). doi:10.1128/JVI.00709-18. PMC 6258936. PMID 30258007.
- ^ a b c Koichi Y, Arvin AM, Campadelli-Fiume G, Mocarski E, Patrick M, Roizman B, Whitley R (2007). Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge, UK: Cambridge University Press. ISBN 978-0-521-82714-0.
- ^ "Panine betaherpesvirus 2 (Chimpanzee cytomegalovirus)". www.uniprot.org. Retrieved 13 March 2019.
- ^ "Simian cytomegalovirus (strain Colburn)". www.uniprot.org. Retrieved 13 March 2019.
- ^ "Macacine betaherpesvirus 3 (Rhesus cytomegalovirus)". www.uniprot.org. Retrieved 13 March 2019.
- ^ "Murid herpesvirus 1, complete genome". 13 August 2018. Retrieved 13 March 2019 – via NCBI Nucleotide.
- ^ a b Sijmons S, Van Ranst M, Maes P (March 2014). "Genomic and functional characteristics of human cytomegalovirus revealed by next-generation sequencing". Viruses. 6 (3): 1049–1072. doi:10.3390/v6031049. PMC 3970138. PMID 24603756.
- ^ Cannon MJ, Hyde TB, Schmid DS (July 2011). "Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection". Reviews in Medical Virology. 21 (4): 240–255. doi:10.1002/rmv.695. PMC 4494736. PMID 21674676.
- ^ Morgan K (3 April 2014). "Plasmids 101: The Promoter Region – Let's Go!". Addgene Blog.
- ^ Reddehase MJ, Lemmermann N, eds. (2006). "Preface". Cytomegaloviruses: Molecular Biology and Immunology. Horizon Scientific Press. pp. xxiv. ISBN 9781904455028.
- ^ Craig JM, Macauley JC, Weller TH, Wirth P (January 1957). "Isolation of intranuclear inclusion producing agents from infants with illnesses resembling cytomegalic inclusion disease". Proceedings of the Society for Experimental Biology and Medicine. 94 (1): 4–12. doi:10.3181/00379727-94-22841. PMID 13400856. S2CID 29263626.
- ^ Chee MS, Bankier AT, Beck S, Bohni R, Brown CM, Cerny R, et al. (1990). "Analysis of the Protein-Coding Content of the Sequence of Human Cytomegalovirus Strain AD169". Cytomegaloviruses. Current Topics in Microbiology and Immunology. Vol. 154. Springer Berlin Heidelberg. pp. 125–69. doi:10.1007/978-3-642-74980-3_6. ISBN 978-3-642-74982-7. PMID 2161319.
- ^ Martí-Carreras J, Maes P (April 2019). "Human cytomegalovirus genomics and transcriptomics through the lens of next-generation sequencing: revision and future challenges". Virus Genes. 55 (2): 138–164. doi:10.1007/s11262-018-1627-3. PMC 6458973. PMID 30604286.
