1,3-dimethyl-2-imidazolidinone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 1,3-dimethyl-2-imidazolidinone | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 5 H 10 N 2 O | ||||||||||||||||||
| Brief description |
clear, colorless liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 114.15 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density | |||||||||||||||||||
| Melting point | |||||||||||||||||||
| boiling point | |||||||||||||||||||
| Vapor pressure |
20 kPa at 25 ° C |
||||||||||||||||||
| solubility |
Fully miscible with water, soluble in dimethylformamide DMF and tetrahydrofuran THF |
||||||||||||||||||
| Refractive index |
1.4720 (25 ° C, 589 nm) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data |
|
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
1,3-Dimethyl-2-imidazolidinone (DMEU) is a cyclic urea with a five-membered imidazolidine skeleton and thus a homologue of the dimethylpropylene urea DMPU. As a high-boiling and strongly polar aprotic solvent with high chemical and thermal stability, DMEU is suitable as a reaction medium for reactions at temperatures above 180 ° C. N, N'-dimethylethylene urea DMEU is miscible with practically all organic solvents and, due to its high dipole moment and high dielectric constant, dissolves many organic and inorganic compounds. It is therefore also a useful substitute for the carcinogenic solvent hexamethylphosphoric triamide HMPT.
Occurrence and representation
1,3-Dimethyl-2-imidazolidinone is produced industrially by reacting phosgene with 1,2-dimethylethylenediamine DMEDA.
With optimized process management - pH control (pH 7.3), control of the temperature and addition of the reactants - DMEU can be obtained in 99.5% purity and 92.1% yield. While avoiding the toxic phosgene, 1,3-dimethyl-2-imidazolidinone can also be obtained with carbon dioxide CO 2 or urea as a source for the carbonyl group in good yield and high purity.
The thermal decomposition of the intermediate product bis-urea, which is not isolated in the continuous process, takes place in 1,3-dimethyl-2-imidazolidinone as a high-boiling solvent and provides DMEU in very high purity (> 99.9%) and very good yield (98%) . The purity of the DMEU obtained is strongly dependent on the purity of the DMEDA used, which often contains by-products with very similar boiling points.
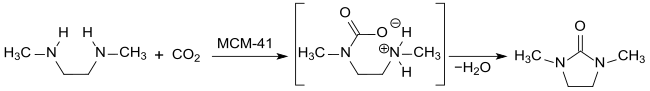
More recently, 1,2-dimethyl-2-imidazolidinone has been discussed as a “sink for the greenhouse gas CO 2 ”. Supercritical carbon dioxide scCO 2 reacts with N, N'-dimethylethylenediamine in the presence of mesoporous silicates of the MCM-41 type at 300 ° C. and 16 MPa pressure in a continuous process quantitatively to form DMEU.
However, the required reaction conditions appear unsuitable for a “green process”.
properties
1,3-Dimethyl-2-imidazolidinone is a clear, colorless liquid with a characteristic pungent odor that mixes with water and many organic solvents. The connection is hygroscopic and has a wide liquid range of over 200 ° C. It is stable to acids and bases even at elevated temperatures. Their high dipole moment (4.05–4.09 D ) and their large dielectric constant (37.60 F · m −1 ) facilitate the solvation of cations , thereby accelerating anionic nucleophilic reactions.
Because of its favorable properties, e.g. B. low skin irritation and low toxicity, DMEU has been used as a substitute for problematic and thermally unstable solvents such. B. Dimethylformamide DMF or dimethyl sulfoxide DMSO suggested. However, in a comparative study of various solvents, DMEU is also classified as “problematic” in addition to acetonitrile , DMSO and 2-methyltetrahydrofuran .
Applications
DMEU as a solvent
1,3-Dimethyl-2-imidazolidinone, which is stable to acids and bases even at high temperatures, is suitable as a solvent for alkalis , which , when mixed with surface-active substances and alcohols, are good cleaning agents for metal and glass surfaces.
With dyes and pigments, DMEU forms stable solutions or dispersions that improve the storage stability and application properties of the preparations.
A solution of sodium naphthalene in 1,3-dimethyl-2-imidazolidinone is suitable for etching polytetrafluoroethylene surfaces for better wetting with adhesives or for connection to metal surfaces.
Like other liquid amides, e.g. B. DMF, NMP or DMPU, DMEU can be used as a stripper , typically with other polar solvents and amines, such as. B. diglycolamine HO- (CH 2 ) 2 -O- (CH 2 ) 2 -NH 2 , can be used for photoresists .
1,2-Dimethyl-2-imidazolidinone is proposed as an extractant for the aromatic mixture BTEX in petroleum refineries instead of the toxic sulfolane .
DMEU as the reaction medium
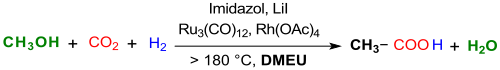
Recently, the synthesis of was acetic acid by methanol - hydrocarboxylation with CO 2 and hydrogen H 2 at 200 ° C in 1,3-dimethyl-2-imidazolidinone as a solvent with a ruthenium - rhodium , -Katalysatorengemisch imidazole as a ligand and lithium iodide LiI as a promoter reported .
With a TOF of a maximum of 30.8 h −1 , a TON of 1022 after five cycles, an acetic acid yield of 70% after 12 hours at 200 ° C and the use of the expensive catalysts trirutheniumdodecacarbonyl Ru 3 (CO) 12 and rhodium (II) -acetat Rh 2 (OAc) 4 , the efficiency of this process is far from that of the Cativa process for acetic acid ( carbonylation of methanol) used on an industrial scale .
3-Phenoxybenzyl alcohol , an important precursor for the insecticide class of pyrethroids , can be produced by the Ullmann reaction of 3-hydroxybenzyl alcohol with chlorobenzene to form 1,3-dimethyl-2-imidazolidinone in the presence of potassium carbonate and catalytic amounts of 8-hydroxyquinoline and copper (I) chloride CuCl can be obtained in 88% yield, while under the same reaction conditions only 21% in DMF and 58% in DMSO are achieved.
The halogen exchange as nucleophilic aromatic substitutions on electron-poor aromatics, such as. B. 4-chlorobenzonitrile, succeeds with potassium fluoride KF in DMEU at 290 ° C in a pressure-tight reactor in 91% yield to 4-fluorobenzonitrile .
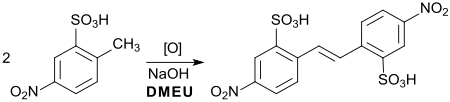
The oxidative dimerization of 2-methyl-5-nitrobenzenesulfonic acid (precursor for azo dyes ) to the corresponding stilbene compound (precursor for the optical brightener 4,4'-diaminostilbene-2,2'-disulfonic acid ), described as early as 1897, can be carried out efficiently with sodium hydroxide in DMEU (Yield 90%).
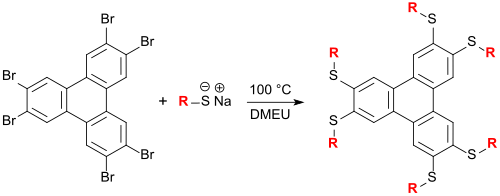
Klaus Praefcke group synthesized a large number of liquid-crystal triphenylene on thioether in DMEU as solvents which columnar can form mesophases.
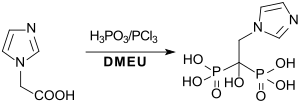
The bisphosphonates risedronic acid and zoledronic acid, which are mainly used against osteoporosis , are available in good yields in homogeneous solution in 1,3-dimethyl-2-imidazolidinone.
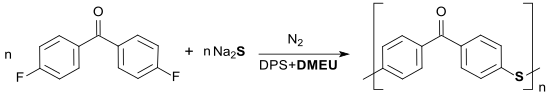
Also polycondensation reactions can be carried out in DMEU or in DMEU-containing solvent mixtures such. B. the formation of high molecular weight poly (p-arylene sulfide ketone) PPSK at temperatures up to 260 ° C. The polymer is only soluble in concentrated sulfuric acid and is stable even at temperatures of 300 ° C.
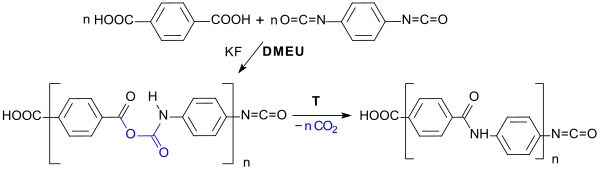
The reaction of aromatic dicarboxylic acids , such as. B. terephthalic acid with a diisocyanate in DMEU as a solvent in the presence of potassium fluoride or the Hünig base DIPEA leads to polyamides with elimination of CO 2 .
Individual evidence
- ↑ a b c d e f g data sheet 1,3-dimethyl-2-imidazolidinone from Sigma-Aldrich , accessed on August 18, 2019 ( PDF ).
- ^ A b c d Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons, 2nd Edition . Elsevier Inc., Oxford, UK 2015, ISBN 978-0-323-28659-6 , pp. 78 .
- ↑ Patent US5594149 : Process for producing 1,3-dialkyl-2-imidazolidinone. Filed April 17, 1996 , published January 14, 1997 , Applicants: Mitsui Toatsu Chemical, Inc., Inventors: H. Naruse, H. Mizuta, S. Umeda, T. Nagata.
- ↑ a b c Entry on 1,3-dimethylimidazolidin-2-one in the GESTIS substance database of the IFA , accessed on August 18, 2016 (JavaScript required)
- ↑ Ellen M. Leahy: 1,3-Dimethyl-2-imidazolidinone . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2001, doi : 10.1002 / 047084289X.rd342 .
- ↑ a b Patent US5872260 : High purity 1,3-dialkylimidazolidinone and preparation process of same. Applied July 21, 1997 , published February 16, 1999 , Applicants: Mitsui Chemicals, Inc., Inventors: H. Mizuta, M. Takaoka, T. Nagata.
- ↑ a b c d B.J. Barker, J. Rosenfarb, JA Caruso: Urea as a solvent in the chemical industry . In: Angew. Chem. Band 91 , no. 7 , 1979, pp. 560-564 , doi : 10.1002 / anie.19790910707 .
- ↑ DMI ™ 1,3-dimethyl-2-imidazolidinones. Mitsui Chemicals, Inc., September 1, 2013, accessed August 18, 2018 .
- ↑ C.-C. Lo, P.-M. Chao: Replacement of carcinogenic HMPA by DMI in insect pheromone synthesis . In: J. Chem. Ecol. tape 16 , no. 12 , 1990, pp. 3245-3253 , doi : 10.1007 / BF00982095 .
- ↑ Patent US4668793 : Process for producing 1,3-dimethyl-2-imidazolidinone. Applied November 1, 1985 , published May 26, 1987 , Applicants: Mitsui Toatsu Chemicals, Inc., Inventors: T. Nagata, N. Kajimoto, M. Wada, H. Nakayama, Y. Yamada.
- ↑ T. Seki, Y. Kokubo, S. Ichikawa, T. Suzuki, Y. Kayaki, T. Ikariya: Mesoporous silica-catalysed continuous chemical fixation of CO 2 with N, N'-dimethylethylenediamine in supercritical CO 2 : the efficient synthesis of 1,3-dimethyl-2-imidazolidinone . In: Chem. Commun. No. 3 , 2009, p. 349-351 , doi : 10.1039 / B817879H .
- ↑ J. Rose color, HL Huffman, Jr., JA Caruso: Dielectric constants, viscosities, and related physical properties of several substituiertem liquid ureas at various Temperatures . In: J. Chem. Eng. Data . tape 21 , no. 2 , 1976, p. 150-153 , doi : 10.1021 / je60069a034 .
- ↑ D. Prat, J. Hayler, A. Wells: A survey of solvent selection guides . In: Green Chem. Band 16 , no. 10 , 2014, p. 4546-4551 , doi : 10.1039 / C4GC01149J .
- ^ Q. Qian, J. Zhang, M. Cui, B. Han: Synthesis of acetic acid via methanol hydrocarboxylation with CO 2 and H 2 . In: Nature Commun. tape 1 , 2016, p. 11481 , doi : 10.1037 / ncomms11481 .
- ↑ R. Oi, C. Shimakawa, S. Takenaka: Ullmann ether synthesis in DMI. Preparation of m-phenoxybenzyl alcohol . In: Chem. Lett. tape 17 , no. 5 , 1988, pp. 899-900 , doi : 10.1246 / cl.1988.899 .
- ↑ H. Suzuki, Y. Kimura: Synthesis of 3,4-difluorobenzonitrile and monofluorobenzonitriles by means of halogen exchange fluorination . In: J. Fluor. Chem. Band 52 , no. 3 , 1991, pp. 341-351 , doi : 10.1016 / S0022-1139 (00) 80348-6 .
- ^ AG Green, AR Wahl: About the oxidation of paranitrotoluene sulfonic acid . In: Ber. German chem. Ges. Band 30 , no. 3 , 1897, p. 3097-3101 , doi : 10.1002 / cber.189703003128 .
- ↑ K. Praefcke, A. Eckert, D. Blunk: Core-halogenated, helical-chiral triphenylene-based columnar liquid crystals . In: Liquid Crystals . tape 22 , no. 2 , 1997, p. 113-119 , doi : 10.1080 / 026782997209478 .
- ↑ Patent US4631143 : Triphenylene derivates. Applied on December 21, 1984 , published on December 23, 1986 , Applicant: Merck Patent GmbH, Inventors: K. Praefcke, B. Kohne, W. Poules, E. Poetsch.
- ↑ Patent WO2008056129A1 : Process for the preparation of biphosphonic acids and salts thereof. Applied on November 6, 2007 , published on May 15, 2008 , applicant: Hovione Inter Ltd., inventor: J. Baptista, Z. Mendes.
- ↑ G.-M. Yan, Z.-M. Li, G. Zhang, H.-H. Ren, S.-S. Yuan, Y. Li, J. Yang: High molecular weight poly (p-phenylene sulfide ketone): synthesis and membrane-forming properties . In: J. Polym. Res. Band 23 , 2016, p. 61 , doi : 10.1007 / s10965-016-0948-y .
- ↑ Patent US5011936 : Process for refining 1,3-dimethyl-2-imidazolidinone. Applied March 7, 1989 , published April 30, 1991 , Applicants: Mitsui Toatsu Chem., Inc., Inventors: T. Kobayashi, M. Wada, S. Obuchi, H. Takayanagi.
- ↑ K.Sasaki, D. Crich: Facile amide bond formation from carboxylic acids and isocyanates . In: Org. Lett. tape 13 , no. 9 , 2011, p. 2256-2259 , doi : 10.1021 / ol200531k .