Atrial fibrillation
| Classification according to ICD-10 | |
|---|---|
| I48.0 | Atrial fibrillation, paroxysmal |
| I48.1 | Atrial fibrillation, persistent |
| I48.2 | Atrial fibrillation, chronic |
| I48.9 | Atrial fibrillation and atrial flutter, unspecified |
| ICD-10 online (WHO version 2019) | |
Atrial fibrillation , the most common form and cause of absolute arrhythmia , is a temporary ( paroxysmal or intermittent ) or permanent (permanent) cardiac arrhythmia with disordered activity of the atria . Common abbreviations are AF or AFib (from english A trial f ibrillation ) VFLI, VoFli, VHF and VHFli .
Atrial fibrillation is the most common significant cardiac arrhythmia, around 300,000 people in Germany alone have atrial fibrillation. Patients fail to notice about 70% of atrial fibrillation attacks. Those affected usually complain of unspecific complaints such as sudden drop in performance, fatigue, palpitations or sleep disorders. Palpitations notice 70–80% of symptomatic patients.
Atrial fibrillation is associated with an increased risk of stroke and heart failure . The mortality ( mortality ) is increased. For most patients, drug or invasive treatment allows a normal or almost normal way of life.
distribution
Atrial fibrillation occurs in 1–2% of the population; In other words, there are around six million affected people in Europe. The prevalence increases from less than 0.5% in the age under 40 to up to 15% in people over 80 years of age. Men are more often affected than women, the lifetime risk for people over 40 years of age is around 25%. The risk of atrial fibrillation increases significantly with the severity of an existing heart disease. With heart failure, atrial fibrillation is found in 30–40% of cases.
causes
Atrial fibrillation can occur without an identifiable cause (idiopathic) or without an identifiable underlying disease (lone atrial fibrillation) . This is the case in about a third of patients, more often with paroxysmal (approx. 45%) than with permanent atrial fibrillation (approx. 25%). About 20-30% of the patients suffer from coronary artery disease , also about 20-30% from arterial hypertension (high blood pressure), almost 20% from a heart valve defect (such as severe mitral valve stenosis ) and about 15% from a heart muscle disease . The most common extracardiac cause of atrial fibrillation in around 0.5–3% of patients is manifest or even latent overactive thyroid gland ( hyperthyroidism , thyrotoxicosis ) with a five- to six-fold increased risk of atrial fibrillation. Atrial fibrillation can also be caused by an electrical accident or occur after operations, especially in the first few days after thoracic surgery (bypass operations, lung resections). Observational studies have shown that alcohol consumption increases the frequency of atrial fibrillation in a dose-dependent manner and is associated with structural changes in the left atrium (enlargement, fibrosis). Also, the sleep apnea syndrome is associated with an increased likelihood of developing atrial fibrillation. One study has shown that sleep apnea therapy reduces the risk of atrial fibrillation recurrence by 42%.
Electrophysiologically , two main mechanisms are held responsible for atrial fibrillation:
- So-called trigger arrhythmias ( atrial extrasystoles and high-frequency focal atrial tachycardias) as triggers, which often have their origin in one of the pulmonary veins , and
- Circular excitations based on anatomical and electrophysiological properties of the atria, which promote the development and maintenance of atrial fibrillation.
Atrial flutter , other supraventricular arrhythmias and the influence of the autonomic nervous system on the heart rate can also lead to atrial fibrillation. Atrial fibrillation itself also leads to “adaptation processes” of the atria (atrial remodeling) , which in turn can maintain atrial fibrillation (“atrial fibrillation maintains atrial fibrillation”).
This "remodeling" affects the electrical, contractile and ultrastructural properties of the atria.
- "Electrical remodeling" is a shortening of the action potential and thus also the atrial refractory period , caused by a reduced influx of Ca 2+ ions into the muscle cells of the atria.
- This reduced Ca 2+ influx also leads to “contractile remodeling”, a loss of contractile strength of the atrial muscles, which persists for some time even after successful cardioversion .
- The weakness of the muscles also promotes an increasing enlargement of the atria, which are already stretched from increased pressure. This enlargement in turn leads to hypertrophy , accelerated cell death and fibrosis in the atrial muscles, which is known as "ultrastructural remodeling".
Only in the last few years have indications of a genetic predisposition for atrial fibrillation been found:
- in 2002 and 2003 was mostly independently autosomal dominant inherited mutation in the gene KCNQ1 described that an increased flow of potassium ions during repolarization leads and promotes the occurrence of atrial fibrillation.
- In 2004 Fox et al. Published the results of a prospective study on 2243 offspring of the patients in the Framingham Heart Study ; this prospective study showed, among other things, a twice as high risk of atrial fibrillation if at least one parent already had atrial fibrillation.
- An increased incidence of AF has been demonstrated for hereditary short and long QT syndrome , for Brugada syndrome and also for some forms of hypertrophic cardiomyopathy . Various other genetic defects have also been described in the meantime.
Disease emergence
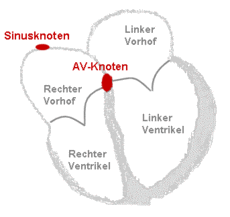
In the normal state, i.e. H. in sinus rhythm , the atria and ventricles of the heart are in immediate succession about 70 times per minute stimulated (see FIG. Structure and conduction system of the heart). The muscle contraction of the atria leads to an additional blood filling of the chambers, which also contract about 150 milliseconds later. This function of the atria is comparable to that of a turbocharger in an engine and increases the stroke volume of the heart chambers by approx. 15%.
In atrial fibrillation, on the other hand, undirected electrical excitations run through the atria. This leads to rapid and disorderly movements of the walls with a frequency of 300 to 600 per minute, the flicker . Below a frequency of 300 per minute it is atrial flutter . A transition area is called atrial fibrillation .
| Schematic representation of the spread of excitation in the heart | ||
| with sinus rhythm | with atrial fibrillation | Legend |
Clinical appearance
The main consequences of atrial fibrillation are:
- Irregular pulse ( absolute arrhythmia or arrhythmia absoluta with pulsus irregularis et inaequalis ). The braking and filtering function of the AV node transfers only around 100 to 160 of the 350–600 atrial impulses per minute to the ventricles at irregular (sometimes also at regular) intervals.
- Too fast pulse ( tachycardia ) in the untreated state. Although the AV node usually protects the ventricles from pulse frequencies of over 200 per minute, the typical frequencies of 100 to 160 per minute are not economical for the heart in the long term and can increase within days to weeks in the case of predisposed patients severe heart muscle weakness with heart failure ( tachycardiomyopathy ). The echocardiographic picture is similar to dilated cardiomyopathy (DCM)
- Heart rate too slow ( bradycardia ), often caused by drugs that slow your heart rate. The slow heartbeat or breaks can be causes of fainting spells ( syncope ). If there is a frequent change between fast and slow phases, one speaks of a bradycardia-tachycardia syndrome.
- Loss of orderly atrial contraction with slight impairment of the heart's pumping capacity. In an otherwise healthy heart, this usually does not matter and is then usually not noticed. If the heart is severely damaged, the lack of atrial contraction can lead to a significant deterioration in exercise capacity.
- Increased risk of embolism . Blood clots ( thrombi ) can easily form in the atria (preferably in the left atrial appendage ) due to the changed blood flow . These thrombi in turn can loosen and then lead to embolic vascular occlusions in the body. The formation of spherical thrombi in the left atrium can also obstruct the outflow of the pulmonary veins, with the possible consequence of pulmonary hypertension . Strokes caused by cerebral embolism are particularly feared . Mesenteric infarcts due to the occlusion of an intestinal vessel are also less common .
- Dizziness or loss of consciousness can occur when the heart stops beating after the end of the seizure- like atrial fibrillation (pre- automatic pause ).
Classification
There are a number of classifications for atrial fibrillation.
After the duration of existence , according to the updated guidelines of the European Society of Cardiology (ESC) in
- first discovered,
- paroxysmal (or intermittent),
- persistent,
- long-lasting persistent and
- permanent atrial fibrillation
assigned. Thereafter, atrial fibrillation is considered paroxysmal if it ends spontaneously within 48 hours to seven days of the suspected onset. It is called persistent if it is stopped by medical or electrical cardioversion and treated with rhythm preservation. Recently, in the updated guideline, atrial fibrillation that has existed for more than a year has been described as persistent if it is to be treated in a rhythm-preserving manner. Atrial fibrillation is classified as permanent if continuation is accepted and no rhythmic treatment is carried out.
In the 2011 version of the ICD-10 code , atrial fibrillation was combined with atrial flutter and not further subdivided. As a result, epidemiological recording in this system is practically no longer possible.
Depending on the heart rate measured in the electrocardiogram (EKG) , atrial fibrillation is also in
- Bradyarrhythmia absoluta (slow form of absolute arrhythmia with a pulse below 60 beats per minute),
- normal frequency absolute arrhythmia (pulse 60 to 100 beats per minute) and
- Tachyarrhythmia absoluta (pulse over 100 beats per minute).
Relevant for the use of new drugs in the context of anticoagulant therapy, but also for rhythm control, is the classification according to the cause into
- valvular , i.e. starting from the mitral valve , and in
- non-valvular atrial fibrillation corresponding to other causes (e.g. high blood pressure).
If the time and situation at which the atrial fibrillation began are known, between
- vagal (usually during the night) and
- sympathetic tone (often during stress, physical exertion or in the morning after getting up)
triggered atrial fibrillation can be distinguished.
EHRA classification
The classification according to the symptoms according to the EHRA score of the European Heart Rhythm Association , based on the NYHA classification for heart failure, is new . It can be used as a decision-making aid when it comes to rhythm-maintaining therapy.
| stage | Severity of symptoms | definition |
|---|---|---|
| EHRA I | No complaints | Normal daily activity is not restricted |
| EHRA II | Slight complaints | |
| EHRA III | Severe complaints | Normal daily activity is restricted |
| EHRA IV | Massive disabling complaints | Normal daily activity is impossible |
Diagnosis
The diagnosis of atrial fibrillation must clarify
- whether there is actually atrial fibrillation,
- whether complications have occurred or are to be expected,
- whether there is an essential underlying or accompanying illness,
- which treatment strategy makes sense and
- whether and what form of anticoagulation ( anticoagulation ) is necessary.
The medical history ( anamnesis ) is indispensable for answering these questions , whereby in particular
- the previous duration of the atrial fibrillation,
- the duration and frequency of previous episodes,
- possibly triggering factors such as alcohol consumption, sleep deficit or operations,
- known heart or thyroid diseases,
- the current symptoms during the arrhythmia and
- previous therapies or attempts at therapy
are important.
Diagnosing atrial fibrillation
Irregular and usually too fast pulse is the main symptom of atrial fibrillation and is almost always detected during the physical examination during palpation and auscultation .
The “atrial waves” ( P waves ) are missing in the ECG , instead there is often an irregular “flicker” of the baseline. Irregularly occurring " ventricular spikes" ( QRS complexes ) can sometimes be better recognized than these fibrillation waves. A regular ventricular action can also rarely occur in atrial fibrillation, for example with AV block III °, in some pacemaker patients or during ventricular tachycardia .
Paroxysmal atrial fibrillation can often only be diagnosed in a long-term ECG ( long-term ECG, usually carried out over 24 hours), by means of event recording (over several days or months) or by an implanted event recorder (over years), as it happens again and again in the meantime There are phases of a normal regular pulse (sinus rhythm) with a normal EKG.
Recognize complications and comorbidities
A heart failure as an important complication of atrial fibrillation is suspected usually based on the patient's symptoms and then in the ultrasound of the heart ( echocardiography confirmed). At the same time, heart valve defects (especially of the mitral valve ), other heart defects or a heart attack can be identified as possible causes of atrial fibrillation during echocardiography . In addition, the likelihood of success of a cardioversion is estimated by assessing the size of the atria. The laboratory blood test is needed to rule out an overactive thyroid or electrolyte disorders . The thromboembolism is probably the most important complication of atrial fibrillation. This involves the loosening of small blood clots from the auricles of the heart with subsequent blockage of arteries (e.g. arteries in the brain resulting in a stroke). Small thrombus formation in atrial fibrillation could also lead to embolic microinfarctions in the brain and the associated faster cognitive decline, at least a new prospective observational study with over 5,000 patients comes to this conclusion.
therapy
The therapy of atrial fibrillation essentially aims at two core problems,
- the treatment of the arrhythmia and
- avoiding embolism .
Treatment of the arrhythmia
When atrial fibrillation occurs for the first time, the aim is to restore and maintain the sinus rhythm. If this is not promising in the long run, heart rate control (by slowing down the ventricular rate and reducing a pulse deficit and treating exacerbation factors ) is the goal.
New atrial fibrillation has a high "self-healing rate". In more than half of the patients, it ends spontaneously within 24 hours (spontaneous cardioversion) . Therefore, treatment during this time can usually be limited to lowering the pulse rate with beta blockers or calcium channel blockers of the verapamil or diltiazem type. If the frequency reduction is not sufficient, Digitalis preparations can be used to inhibit AV conduction . The use of class I antiarrhythmics is less necessary. Factors that can be influenced that favor atrial fibrillation are eliminated as far as possible. High blood pressure values, electrolyte disorders, circulatory disorders of the heart and an overactive thyroid should be considered. Weight loss can also improve atrial fibrillation.
If atrial fibrillation persists, two different therapy strategies are possible:

- Rhythm control: Drug or electrical cardioversion is used to attempt to restore normal sinus rhythm. If atrial fibrillation occurs again ( relapse ), cardioverting is carried out again, and antiarrhythmics (e.g. amiodarone , flecainide ) or beta blockers are often prescribed to avoid relapses . If the outcome is unsatisfactory during drug therapy, sclerotherapy ( ablation ) of parts of the inner lining of the heart (endocardium) can be considered, which is either performed surgically (rarely performed) or as part of a cardiac catheter procedure (catheter ablation). Either longitudinal lesions are placed in the area of the left atrium or muscle bundles are removed in a ring at the mouths of the pulmonary veins ( pulmonary vein isolation ). These procedures are time-consuming (long intervention times) and burdened by possible complications (especially thromboembolism and pulmonary vein stenosis). The long-term results are inconsistent, and an improvement can be expected in 50–80% of patients. Attempts to suppress the occurrence of atrial fibrillation by means of pacemaker therapy are also still experimental ; in some patients with paroxysmal atrial fibrillation, special stimulation techniques lead to a significant reduction in the frequency of seizures.
- Rate control: The atrial fibrillation is left in place, so no cardioversion attempt is made. The optimal target heart rate in patients with atrial fibrillation is unclear. ESC guidelines recommend a heart rate <110 beats / minute at rest, unless the symptoms require more stringent rate control. Suitable drugs for frequency control are beta blockers, digitalis, diltiazem or verapamil.
Since these strategies have proven to be prognostically equivalent in several large studies , the extent of the patient's complaints (the clinical symptoms ) is now the decisive criterion for the choice of therapy.
Embolic prophylaxis, CHADS 2 score
If atrial fibrillation lasts longer than 48 hours, the risk of blood clots ( thrombi ) forming increases, especially in the left atrium of the heart. These thrombi can loosen, be transported with the bloodstream to remote vessels and cause acute vascular occlusions ( embolisms ) there. It is critical that the sick do not feel normofrequent atrial fibrillation, which usually lasts for a long time, and only consult a doctor in a tachycardiac phase. Conversion therapy harbors the dangers of thromboembolism, which is why this risk should be reduced beforehand by means of TEE (transesophageal echocardiography, "swallowing echo") or by drug-based inhibition of blood clotting ( anticoagulation ).
This drug-based inhibition of blood clotting (anticoagulation, also incorrectly referred to as "blood thinning") is also considered in the case of persistent atrial fibrillation; In addition, after a successful cardioversion, anticoagulation is necessary for about four weeks, because the atrium can continue to show what is known as “stunning” with reduced contraction for so long and atrial thrombi can continue to form despite the sinus rhythm.
The majority of patients receive acetylsalicylic acid (ASA) or coumarins in tablet form, in Austria, Germany and Switzerland mostly phenprocoumon . The previously necessary weighing of the advantages of this therapy (rarer embolisms, especially fewer strokes ) with the possible disadvantages (increased bleeding tendency) requires the most accurate possible knowledge of the individual embolism risk. This risk increases statistically by 2.5 times for previous embolisms, with age by 1.4 times per decade of life, 1.6 times for patients with high blood pressure , 1.5 times for coronary patients and 1.7 times for diabetics .
An alternative therapy in the prevention of thromboembolism set the "new oral anticoagulants" [NOAK] as the oral direct thrombin inhibitor dabigatran etexilate or oral Factor Xa inhibitors rivaroxaban , Edoxaban and apixaban represents, in contrast to the standard therapy with coumarins a greater therapeutic breadth comprise and are easier to use since coagulation monitoring is not required. This is intended to reduce the risk of bleeding complications with the same or better effectiveness. The four substances mentioned were clinically tested in large-scale phase III studies. Further active ingredients are currently in clinical trials.
On the other hand, the risk of bleeding increases only slightly with age. The therapy decision is based on the following joint recommendations of the American Heart Association (AHA), the American College of Cardiology (ACC) and the European Society of Cardiology (ESC) from 2006.
| Recommendation classes and evidence levels in ACC / AHA / ESC format | |
|---|---|
| Recommendation class (Class) | |
| I. | Evidence or general agreement that the recommendation is beneficial, useful and effective. |
| II | Contradicting evidence or opinions about the benefit and effectiveness with either a predominantly positive (Class IIa) or less well established (Class IIb) assessment. |
| III | Evidence or general agreement that the therapy is not useful, effective and may be harmful. |
| Level of evidence (level of evidence) | |
| A. | Data from multiple randomized studies or meta-analyzes. |
| B. | Data from a randomized study or non-randomized research. |
| C. | Expert opinion, case studies or established standard of therapy. |
- With the exception of patients with lone atrial fibrillation (before the age of 60 without heart disease) and those with contraindications , anticoagulant therapy is recommended for all patients with atrial fibrillation (Class I, Level of Evidence: A) .
- The type of coagulation inhibition (ASA or anticoagulation) should be determined individually for each patient depending on the risk of a stroke on the one hand and bleeding on the other (Class I, Level of Evidence: A) .
- Patients with a high risk of stroke (who have already suffered an embolism or rheumatic mitral valve stenosis ) without an artificial heart valve and without contraindications should receive vitamin K antagonists in a dose that leads to an INR of 2.0 to 3.0 (Class I, Level of Evidence: A) .
- Anticoagulation with vitamin K antagonists is recommended for patients with more than one "moderate" risk factor (age over 74 years, high blood pressure, heart failure, restricted pumping function of the left ventricle with an ejection fraction of less than 35% or diabetes mellitus) (Class I , Level of Evidence: A) . If only one of these risk factors is present, ASA or a vitamin K antagonist can be used depending on the risk of bleeding, the reliability of the anticoagulation and the patient's request (Class IIa, Level of Evidence: A) . The same applies to patients with at least one of the less well-validated risk factors: coronary artery disease, age over 64 years or female gender (Class IIa, Level of Evidence: B) .
- As an alternative to the vitamin K antagonists, ASA in a dose of 81 to 325 mg daily is recommended for contraindications and for patients with a low risk of stroke (Class I, Level of Evidence: A) .
- During therapy with vitamin K antagonists, the INR should be checked at least once a week in the setting phase and at least once a month if the setting is stable (Class I, Level of Evidence: A) .
- Anticoagulation in patients with mechanical heart valve prostheses with an INR of at least 2.5 is based on the prosthesis type (Class I, Level of Evidence: B) .
- It is justifiable to select the anticoagulant therapy regardless of the type of atrial fibrillation (paroxysmal, persistent or permanent) (Class IIa, Level of Evidence: B) .
- Interrupting anticoagulation for up to seven days without replacement by heparin for diagnostic or surgical interventions with an increased risk of bleeding is sensible in patients without a mechanical heart valve prosthesis (Class IIa, Level of Evidence: C) . In the event of an interruption of more than a week, unfractionated or low molecular weight heparin can be administered as a bridging measure, even if the effectiveness of this method is uncertain in this situation (Class IIb, Level of Evidence: C) .
- Patients with atrial fibrillation after PTCA or bypass surgery can receive ASA (less than 100 mg per day) and clopidogrel (75 mg per day) in addition to anticoagulation, although this is associated with an increased risk of bleeding and has not been thoroughly investigated (Class IIb, Level of Evidence: C) . In connection with PTCA, anticoagulation can be temporarily interrupted, but should be continued as early as possible (Class IIb, Level of Evidence: C) .
- Long-term anticoagulation is not recommended in patients under 60 years of age without heart disease or other risk factors for embolism (Class III, Level of Evidence: C) .
|
CHADS 2 score for estimating the risk of stroke in atrial fibrillation |
||
|---|---|---|
| If there is ... | … surrendered | |
| C ( congestive heart failure ) | Structural heart disease that causes heart failure |
1 point |
| H (hypertension) | Arterial hypertension (also treated) |
1 point |
| A (age) | Age ≥ 75 years | 1 point |
| D (diabetes) | Diabetes mellitus | 1 point |
| S (stroke) | Had a stroke or transient ischemic attack |
2 points |
CHADS 2 score
The so-called CHADS 2 score, which is also recommended in a slightly modified form in the current guidelines of the European Society of Cardiology, is helpful for the simple assessment of the stroke risk in atrial fibrillation . If the score = 0, i.e. there is no risk factor for a stroke, the risk of severe bleeding outweighs the risk and prophylaxis with acetylsalicylic acid (ASA, 100–300 mg / day) should be used. With a score = 1, individual weighing must be carried out on a case-by-case basis (depending on the severity and frequency of the atrial fibrillation, severity of the risk factors, etc.) and with a score> 1, anticoagulation with coumarins should be carried out (INR 2–3).
Even with appropriate treatment with coumarins, brain embolism is quite common in people at risk. Using sensitive magnetic resonance imaging , cerebral infarctions were detected in 21% of high-risk patients within three years, but more than half of them caused no symptoms. A high risk of embolism was found in patients with dense spontaneous echoes in the left atrium, with a slow blood flow rate in the left atrial appendage, and in those who had previously suffered an embolism. Of the patients without any of these characteristics, only seven percent suffered a brain embolism in the three years.
| CHA 2 DS 2 -VASc score (further development of the CHADS 2 score) | ||
|---|---|---|
| If there is ... | … surrendered | |
| C (congestive heart failure) | Structural heart disease that causes heart failure |
1 point |
| H (hypertension) | Arterial hypertension (also treated) |
1 point |
| A 2 (age) | Age> 75 years | 2 points |
| D (diabetes) | Diabetes mellitus | 1 point |
| S 2 (stroke) | Had a stroke or transient ischemic attack |
2 points |
| V (vascular disease) | z. B. had a heart attack, PAD | 1 point |
| A (age) | Age 65-74 | 1 point |
| Sc (sex category) | female gender | 1 point |
CHA 2 DS 2 -VASc score
For some time now, especially in Europe, a modification of the CHADS 2 score, the CHA 2 DS 2 -VASc score, has been used for risk assessment, which may allow better risk stratification by better differentiating people with low risk. In this modification, additional points are awarded for existing vascular diseases and female gender. In addition, the age is differentiated more precisely (from 65 years one point, from 75 years another point). As a special feature, it should be taken into account that in women under 65 without further risk factors, the female gender is not included in the score as an independent risk (0 points).
Individuals who achieve at least two points in the CHA 2 DS 2 -VASc score should receive oral anticoagulant medication with coumarins. The added benefit was controversially discussed in a study published in 2010.
Anticoagulation and risk of bleeding
To assess the risk of cerebral hemorrhage under anticoagulation, the European Society of Cardiology presented a risk score in 2010 , the HAS-BLED score . The acronym "has bled" means "has bled".
| HAS-BLED score (ESC guidelines 2010) | ||
|---|---|---|
| Digit | clinic | Points |
| H | Hypertension (RR systolic over 160 mmHg) | 1 |
| A. | Severe liver / kidney dysfunction (1 point each) | 1-2 |
| S. | History of stroke | 1 |
| B. | Have had any bleeding or have a tendency to bleed | 1 |
| L. | Unstable setting (<60% of the INR values in the target range) | 1 |
| E. | Age over 65 years | 1 |
| D. | Drugs such as NSAIDs or alcohol abuse | 1-2 |
From a score = 3, there is a relevant risk of bleeding, which requires particular caution when prescribing anticoagulants.
The risk factors for bleeding overlap e.g. T. with those for embolism (hypertension, previous stroke, age). Patients with a high HAS-BLED score i. d. Usually also at high risk of stroke. Therefore, a higher HAS-BLED score should not be understood as an absolute contraindication for anticoagulation, but v. a. Also as a hint to optimize influenceable risk factors (labile INR setting, concomitant medication).
Surgical embolic prophylaxis
In patients with an increased risk of bleeding (HAS-BLED score> 3) or a contraindication to anticoagulants, it is possible to minimize the risk of embolism by occluding the left atrial appendage, which is a typical source of embolism. For this purpose, an atrial implant ( umbrella ) is advanced to the left atrium with the help of a catheter through the puncture of a large vein by piercing the atrial septum . There the implant is unfolded and placed directly at the access to the atrial appendage. In the following months, the atrial umbrella grows in and is completely covered by the inner lining of the heart . The atrial appendage is thus permanently closed, blood clots can no longer form there. Instead of taking an anticoagulant, it is sufficient to take platelet aggregation inhibitors in the long term . Previous studies have shown that embolic prophylaxis is not inferior to oral anticoagulation. A study published in 2009 presented a significant risk to this procedure. Every 20th patient developed a pericardial effusion , every 50th patient required open heart surgery and 1% of patients suffered a stroke caused by an air embolism or a blood clot.
Minimally invasive catheter ablation
Catheter ablation is being considered more and more . Using a special catheter, a scar is placed in the inner wall of the heart via the inguinal veins in order to stop the electrical impulses that trigger atrial fibrillation. The scar is mainly inflicted on the heart by heat, but there are now also procedures that work with cold or laser. Ablation provides good results especially in patients with paroxysmal atrial fibrillation. However, in some cases the ablation must be performed multiple times. Ablation is also an option in young people to avoid lifelong medication associated with side effects. This minimally invasive procedure is relatively low-risk, but occasionally more severe complications such as pericardial tamponade or pulmonary embolism occur.
Permanent freedom from disease is not guaranteed even after catheter ablation.
The increasing tendency to perform such interventions also met with criticism from some medical specialists. In a review article in medicines letter of November 2014, a team of authors came to the personal conclusion that one should catheter ablation only in highly symptomatic patients whose symptoms could not be sufficiently alleviated with Class I and Class III antiarrhythmic drugs, considering. A mere patient wish not to take any more medication would not be a reason for performing an ablation. The risks of the procedure should also be clearly pointed out. Literally it said: "Representations that ablation is an uncomplicated routine procedure with a high success rate are untenable."
However, there are more and more recent studies that demonstrate the superiority of catheter ablation in comparison to drug rhythm control in clinical studies , so that there is already talk of a paradigm shift from drug therapy to catheter ablation. This could currently be shown in a multicenter study ("CASTLE-AF") in 363 patients with atrial fibrillation and heart failure from NYHA II and more, in which, after a median follow-up of 38 months, 28.5% of the patients opposed catheter ablation 44.6% of the drug-treated patients had died or had to be hospitalized because of heart failure (hazard ratio HR = 0.62), with death in 13.4% against 25.0% (HR = 0.56), although only despite catheter ablation 63% of the patients were in sinus rhythm after 60 months (against 22% in the drug group). During catheter ablation, there were seven serious, non-fatal complications (4%), including three bleeds requiring a transfusion , three pericardial effusions (with one pericardial puncture ) and one asymptomatic pulmonary vein stenosis.
Special situations
Perioperative atrial fibrillation
Especially after operations on heart Atrial fibrillation is a common complication . It occurs, depending on the procedure performed and previous damage to the heart, with a frequency of 10–77%, usually on the second day after the operation. It is more common for interventions on the mitral valve (up to 73%) than for bypass operations (10–33%). Other risk factors include old age, an enlarged left atrium, long surgery times, high blood pressure and previous atrial fibrillation. The prognosis for perioperative atrial fibrillation is good: the sinus rhythm can be restored in around 90% of patients and an impairment of the probability of survival could not be determined. Potassium and magnesium infusions as well as beta blockers and other antiarrhythmics are used to avoid perioperative atrial fibrillation .
Atrial fibrillation and stimulants - Holiday Heart Syndrome
In some people, including those with healthy hearts, atrial fibrillation can be provoked by alcohol . In some studies, more than half of the cases of paroxysmal atrial fibrillation had occurred after alcohol consumption. Typically, the arrhythmia begins a few hours after taking an unusually high dose of alcohol, often in the second half of the night, on the weekend or after physical exertion. For this constellation, especially observed in younger men, the term “Holiday Heart Syndrome” was coined based on a publication from 1983 . In almost all patients, the arrhythmia ends within 24 hours without special treatment. In the Danish Diet Cancer and Health Study published in 2004, men with an alcohol consumption of more than 20 g per day were found to have a 44% increased risk of atrial fibrillation. Men who drank 12 g or less per day were at normal risk, as were women. In a controlled study from Australia it was also shown that in patients who regularly consumed alcohol, the recurrence of atrial fibrillation can be reduced by largely abstaining from alcohol. The medicinal product letter therefore recommends abstinence from alcohol in the case of paroxysmal atrial fibrillation. This is more tolerable than increasing the dose of antiarrhythmic drugs or costly ablation procedures.
The consumption of coffee or tea , which used to be seen as a risk, on the other hand, turned out to be harmless in the same study in connection with atrial fibrillation. It even occurred a little less often with regular consumption.
Prospect of healing
The mortality in atrial fibrillation is about twice as high as in people of the same age with a normal heart rhythm, which is mainly or exclusively due to the more frequent heart diseases. On average, around six percent of patients with atrial fibrillation suffer a stroke each year, and 15–20% of all strokes occur with atrial fibrillation.
In 2006, approximately 2.5 million Americans and 4.5 million EU citizens are expected to have atrial fibrillation. The costs caused by atrial fibrillation are estimated at EUR 13.5 billion annually for the EU countries. The number of hospital stays for atrial fibrillation increased by 66% from 1996 to 2006.
Occurrence in animals
For dog and cat Atrial fibrillation occurs at frequencies up to 700 beats per minute on. While it can be seen regularly in dogs, cats are rarely affected.
dog
In dogs, members of large breeds are most frequently affected, with the resulting heartbeat rates of over 230 beats per minute. The explanation for the formation can be seen in principle in the same mechanisms as explained above. In addition, there is the theory of the “critical mass” of the heart. Small breeds with correspondingly small hearts are far less likely to develop atrial fibrillation than larger dogs. Atrial fibrillation can occur in some giant breeds without a detectable underlying disease. Usually, however, it is secondary to an enlargement of the atria of the heart. The stretching of the atrial walls may have an influence here. The "electrical remodeling" already mentioned above seems to be pronounced in dogs, as once atrial fibrillation can no longer be eliminated in many animals.
Atrial fibrillation is most common as a secondary occurrence in dogs with the underlying disease dilated cardiomyopathy . Other possible triggers are diseases with chronic volume overload of the heart ( mitral valve insufficiency, persistent ductus arteriosus that has not been resolved ). Idiopathic atrial fibrillation is seen relatively rarely . Coronary heart disease or high blood pressure rarely occur in dogs in contrast to humans and are therefore negligible as causes.
The goal of therapy is to reduce the heart rate to 100–140 per minute at rest. In addition to treating the underlying disease, which in the case of dilated cardiomyopathy is the most common trigger in the administration of pimobendan in dogs, drugs such as digitalis , atenolol or diltiazem are administered in dogs and cats to reduce the heart rate. Embolism prophylaxis is not required in dogs.
cat
In cats atrial fibrillation is much more rarely found, even here in substantially the result of an excessive increase in the atria, which are usually on a cardiomyopathy (usually hypertrophic, restrictive rarely, very rarely dilated) is based. The resulting heart rate can reach 320 per minute. The drugs described above are used therapeutically. In addition, embolism prophylaxis (usually with acetylsalicylic acid ) is absolutely necessary to avoid the high risk of embolism in cats of the end of the aortic branch (so-called riding aortic thrombus , → ischemic myopathy in cats ), the renal vessels , the mesenteric vessels ( subclavian artery ) or the lower clavicle artery and caudal artery to avoid.
literature
- AJ Camm u. a .: 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: An update of the 2010 ESC Guidelines for the management of atrial fibrillation * Developed with the special contribution of the European Heart Rhythm Association. In: European Heart Journal . Volume 33, Number 21, November 2012, pp. 2719-2747, ISSN 1522-9645 . doi: 10.1093 / eurheartj / ehs253 . PMID 22922413 .
- Hans-Joachim Trappe: Atrial fibrillation - the certain and the new . In: Dtsch Arztebl Int . No. 109 (1-2) , 2012, pp. 1–7 ( review article ).
- A. Schuchert et al .: Commentary on the ACC / AHA / ESC guidelines 2001 on the prevention of arterial thromboembolism in patients with atrial fibrillation . Z Kardiol (2003) 92: 694-703. text
- E. Hoffmann, S. Janko, C. Reithmann, G. Steinbeck: Trigger mechanisms of atrial fibrillation. In: Journal of Cardiology. Volume 91, Number 1, January 2002, pp. 24-32, ISSN 0300-5860 . PMID 11963204 .
- Fox / Sisson / Moïse: Textbook of Canine and Feline Cardiology. 2nd Edition. Saunders, 1999, ISBN 0-7216-4044-3 , pp. 331-391.
- Wilhelm Haverkamp: Atrial fibrillation up to date: diagnosis / therapy / treatment of special cases . close2real Verlag, Berlin 2018, ISBN 978-3-00-058868-6 .
Web links
- Competence Network Atrial Fibrillation - National association of internists, cardiologists and cardiac surgeons for the development of new methods in the diagnosis and treatment of atrial fibrillation
Individual evidence
- ↑ Mewis, Riessen, Spyridopoulos (ed.): Cardiology compact - Everything for ward and specialist examination . 2nd Edition. Thieme, Stuttgart / New York 2006, ISBN 3-13-130742-0 , pp. 532, 535, 536 .
- ↑ Alan John Camm et al. a .: 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: An update of the 2010 ESC Guidelines for the management of atrial fibrillation * Developed with the special contribution of the European Heart Rhythm Association. In: European Heart Journal . Volume 33, Number 21, November 2012, pp. 2719-2747, ISSN 1522-9645 . doi: 10.1093 / eurheartj / ehs253 . PMID 22922413 . (English)
- ↑ a b c d A. John Camm, et al .: Guidelines for the management of atrial fibrillation . In: European Heart Journal . tape 31 , 2010, p. 2369–2429 , doi : 10.1093 / eurheartj / ehq278 ( online [PDF]). Guidelines for the management of atrial fibrillation ( Memento of the original dated November 17, 2011 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Harald Feldmann, Tilman Brusis: The opinion of the ear, nose and throat doctor . Georg Thieme Verlag, 2012, ISBN 978-3-13-160047-9 ( google.com [accessed on May 31, 2016]).
- ^ Voskoboinik A. et al .: Alcohol and Atrial Fibrillation: A Sobering Review . In: Journal of the American College of Cardiology . tape 68 , no. 23 , 2016, p. 2567 ( sciencedirect.com ).
- ↑ Sleep apnea atrial fibrillation - OSAS treatment can help. In: SeegartenKlinik Heidelberg. February 4, 2020, accessed on May 11, 2020 (German).
- ↑ U. Schotten et al .: Atrial fibrillation: Basic research provides new therapeutic approaches . Deutsches Ärzteblatt (2006) 103: B1491 – B1497.
- ^ Herbert Reindell , Helmut Klepzig: Diseases of the heart and the vessels. In: Ludwig Heilmeyer (ed.): Textbook of internal medicine. Springer-Verlag, Berlin / Göttingen / Heidelberg 1955; 2nd edition ibid. 1961, pp. 578-580 ( Hypertension in the large and small circulation ).
- ↑ a b P Kirchhoff, et al .: Commentary on the ESC guidelines on atrial fibrillation . In: The cardiologist . 6, February 2012, pp. 12-27. doi : 10.1007 / s12181-011-0395-2 .
- ^ EL Thacker, B. .. McKnight, BM Psaty, WT Longstreth, CM Sitlani, S. .. Dublin, AM Arnold, AL Fitzpatrick, RF Gottesman, SR Heckbert: Atrial fibrillation and cognitive decline: A longitudinal cohort study. In: Neurology. , S., doi: 10.1212 / WNL.0b013e31829a33d1 .
- ^ Anne Paschen: Heart. In: Jörg Braun, Roland Preuss (Ed.): Clinic Guide Intensive Care Medicine. 9th edition. Elsevier, Munich 2016, ISBN 978-3-437-23763-8 , pp. 185–283, here: p. 230 ( frequency control ).
- ↑ Hany S. Abed, Gary A. Wittert et al. a .: Effect of Weight Reduction and Cardiometabolic Risk Factor Management on Symptom Burden and Severity in Patients With Atrial Fibrillation. In: JAMA. 310, 2013, p. 2050, doi: 10.1001 / jama.2013.280521 .
- ↑ ESC Pocket Guideline: Management of Atrial Fibrillation , Version 2016.
- ↑ Stuart J. Connolly et al .: Dabigatran versus warfarin in patients with atrial fibrillation. In: The New England journal of medicine . Volume 361, Number 12, September 2009, pp. 1139-1151, ISSN 1533-4406 . doi: 10.1056 / NEJMoa0905561 . PMID 19717844 .
- ↑ Manesh R. Patel et al .: Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. In: The New England Journal of Medicine . Volume 365, Number 10, September 2011, pp. 883-891, ISSN 1533-4406 . doi: 10.1056 / NEJMoa1009638 . PMID 21830957 .
- ↑ CB Granger, et al .: Apixaban versus warfarin in patients with atrial fibrillation. In: The New England Journal of Medicine . Volume 365, Number 11, September 2011, pp. 981-992, ISSN 1533-4406 . doi: 10.1056 / NEJMoa1107039 . PMID 21870978 .
- ^ A b V. Fuster et al .: ACC / AHA / ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology / American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J Am Coll Cardiol (2006) 48: e149–246. PMID 16904533 .
- ↑ a b American College of Cardiology; American Heart Association Task Force; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association; Heart Rhythm Society et al .: ACC / AHA / ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology / American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. In: Europace. Volume 8, Number 9, September 2006, pp. 651-745, ISSN 1099-5129 . doi: 10.1093 / europace / eul097 . PMID 16987906 .
- ↑ P. Bernhardt, H. Schmidt, T. Sommer, B. Lüderitz, H. Omran: Atrial fibrillation - patients at high risk for cerebral embolism. In: Clinical research in cardiology. Volume 95, Number 3, March 2006, pp. 148-153, ISSN 1861-0684 . doi: 10.1007 / s00392-006-0344-4 . PMID 16598527 .
- ↑ Lip GYH: Improving stroke and thromboembolism risk stratification in chronic atrial fibrillation . E-Journal of the ESC Council for Cardiology Practice, Vol. 8, N ° 36, June 9, 2010
- ↑ Laurent Azoulay, Teresa Simon, Sophie Dell'Aniello, Christel Renoux, and Samy Suissa Abstract 18044: Comparison of the CHADS 2 and CHA 2 DS 2 -VASc Scores in Predicting Stroke Events in Patients With Atrial Fibrillation Circulation 122: A18044 / 2010
- ↑ F. Meincke, K.-H. Kuck, MW Bergmann: Interventional atrial appendage closure. Alternative to anticoagulation in stroke prophylaxis for atrial fibrillation ; in: Herz No. 38, 2013 ISSN 0340-9937 , pp. 239-246
- ↑ [1] Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomized non-inferiority trial, Abstract, The Lancet August 2009, accessed February 28, 2014
- ↑ Catheter ablation - Atrial Fibrillation Competence Network . Website of the network headquarters at the Münster University Hospital. Retrieved August 29, 2014.
- ↑ Atrial ablation for atrial fibrillation. In: The medicament letter . November 2014, accessed on April 27, 2017 (Volume 48, No. 11, November 2014).
- ↑ Mark S. Link: Paradigm shift for Treatment of atrial fibrillation in heart failure . New England Journal of Medicine 2018, Volume 378, Issue 5 February 1, 2018, pages 468-469; DOI: 10.1056 / NEJMe1714782
- ↑ Nassir F. Marrouche, Johannes Brachmann, Dietrich Andresen, Jürgen Siebels, Lucas Boersma, Luc Jordaens, Béla Merkely, Evgeny Pokushalov, Prashanthan Sanders, Jochen Proff, Heribert Schunkert, MD, Hildegard Christ, Jürgen Vogt, Dietmar Bänsch for the CASTLE AF Researcher: Catheter Ablation for Atrial Fibrillation with Heart Failure . New England Journal of Medicine 2018, Volume 378, Issue 5, Feb. 1, 201, pages 417-427; DOI: 10.1056 / NEJMoa1707855
- ↑ Voskoboinik A et. al .: Alcohol Abstinence in Drinkers with Atrial Fibrillation . In: New England Journal of Medicine . tape 382 , 2020, p. 20-28 .
- ↑ Fewer recurrences of atrial fibrillation with less alcohol consumption? In: Ludwig WD, Schuler J (ed.): The drug letter . tape 54 , 2020, p. 11 .