Ammonium nitrate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ammonium nitrate | |||||||||||||||
| other names |
|
|||||||||||||||
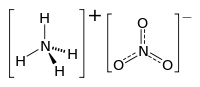
| Molecular formula | NH 4 NO 3 | |||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 80.04 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.72 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
169 ° C |
|||||||||||||||
| boiling point |
210 ° C (15 h Pa ), at normal pressure decomposition from 170 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−366 kJ mol −1 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ammonium nitrate is the salt that is formed from ammonia and nitric acid . It is used in particular in the manufacture of fertilizers and explosives .
history
Ammonium nitrate was first produced in 1659 by Johann Rudolph Glauber by reacting ammonium carbonate with nitric acid. It was not until the beginning of the 19th century that Grindel and Robin considered it as a substitute for potassium nitrate in black powder for use in explosives. Its explosive properties were reported by Reise and Millon in 1849 when a mixture of powdered ammonium nitrate and charcoal exploded when heated. At the time, ammonium nitrate was not considered an explosive, although small fires and explosions involving ammonium nitrate have occurred around the world. At the end of the 19th century, attempts were made in Europe to limit the use of black powder in coal mines and to find a safe alternative to explosives. In England, after experimenting with various explosives, several were eventually recommended, most of which were based on ammonium nitrate. Both dynamite and black powder failed the tests and were replaced with explosives based on ammonium nitrate. The results obtained by this committee led to the British Coal Mining Ordinance of 1906. By 1913, British coal production reached an all-time high of 287 million tons, using more than 5,000 tons of explosives annually, of which from 1917 92% of that amount was based on ammonium nitrate. To reduce the cost of explosive compositions, the explosives industry added more of the cheaper compound ammonium nitrate to the formulations, but this had the unfortunate side effect of increasing the explosives' sensitivity to moisture. Chemists overcame this problem by coating the ammonium nitrate with various inorganic powders, mixing it with dynamite, and by improving the packaging of the explosives to prevent water ingress.
After the end of the First World War, a BASF chemical plant in Oppau produced large quantities of ammonium nitrate as a fertilizer. In order to improve the hygroscopic properties of ammonium nitrate , which are unfavorable for use as fertilizer , potassium chloride was initially added, which resulted in a conversion into a mixture of ammonium chloride and potassium nitrate, which was referred to as potassium ammonium nitrate. The potassium chloride was later replaced by ammonium sulfate , creating a product called ammonium sulfate nitrate (chemically ammonium sulfate nitrate ). The term Leuna nitrate, which refers to BASF's Leuna factory, is sometimes used as a synonym for ammonium sulphate nitrate. The ammonium sulphate nitrate produced in Oppau was often referred to simply as mixed salt. This product is a source of both primary (nitrogen) and secondary (sulfur) plant nutrients. The mixture of the two compounds forms either just a mixture of the two compounds or double salts (2AN-AS or 3AN-AS), depending on the manufacturing process and mixing ratios. The formation of double salts from AN and AS has been known since 1909 from the work of the Dutch physical chemist Franciscus AH Schreinemakers (1864–1945). On the morning of September 21, 1921, two explosions in quick succession of around 400 tons of ammonium sulfate nitrate occurred in the Oppau plant, killing more than 500 people. This explosion resulted in extensive investigations into the accident and studies on the properties of ammonium sulfate nitrate but also ammonium nitrate.
After the end of World War II, the U.S. government began shipping to Europe what is known as fertilizer grade ammonium nitrate (FGAN), which consisted of granular ammonium nitrate coated with about 0.75% wax and conditioned with about 3.5% clay was. Since this material was not considered an explosive, no special precautions were taken in handling or transporting it - workers even smoked while the material was being loaded. Numerous transports were carried out without difficulty until April 16 and 17, 1947, until a terrible explosion occurred. The SS Grandchamp and SS Highflyer, both anchored in Texas City harbor and loaded with FGAN, exploded. As a result of these disasters, a number of investigations were launched in the United States to determine the possible causes of the explosions. At the same time, a more thorough study of the explosive properties of ammonium nitrate and its mixtures with organic and inorganic materials was also carried out. No sooner had the explosion occurred in Texas City than a similar explosion on board the SS Ocean Liberty rocked the port of Brest in France on July 28, 1947 . The investigations showed that ammonium nitrate was much more dangerous than previously thought and stricter regulations regulating its storage, loading and transport in the USA were immediately put into effect.
In the 1970s, US companies Ireco and DuPont began adding aluminum and monomethylamine nitrate (MAN) to their formulations to make gelled explosives that could more easily explode. More recent developments concern the production of emulsion explosives (sometimes with mixtures of ammonium and sodium nitrate ), which contain droplets of a solution of ammonium nitrate in oil.
Manufacturing
Ammonium nitrate (NH 4 NO 3 ) is formed by neutralization of ammonia with nitric acid .
The reaction is strongly exothermic with a heat of reaction of −146 kJ mol −1 .
properties
Physical Properties
Ammonium nitrate forms colorless, hygroscopic crystals that melt at 169.6 ° C. The solid can exist in five different polymorphic crystal forms, with transition temperatures at −16.9 ° C, 32.3 ° C, 84.2 ° C and 125.2 ° C. The first two phase changes near room temperature are responsible for the tendency of ammonium nitrate crystals to stick together. The polymorphic forms appear in different crystal lattices:
Crystal lattice of modifications Polymorph I 169.6 ° C ... 125.2 ° C cubic Polymorph II 125.2 ° C ... 84.2 ° C tetragonal Polymorph III 84.2 ° C ... 32.2 ° C orthorhombic Polymorph IV 32.2 ° C ... −16.9 ° C orthorhombic Polymorph V <−16.9 ° C tetragonal
The phase transition between polymorphs IV and III at 32.2 ° C is relevant when handling, but also when storing the substance. In formulations for fertilizers or explosives, this behavior can lead to undesired changes in the morphology and thus the properties. This phase transition can be suppressed by doping with various salts in order to obtain so-called phase-stabilized ammonium nitrate. Suitable salts can be various potassium salts, such as potassium fluoride , potassium chloride , potassium nitrate , potassium carbonate , potassium sulfate , potassium rhodanide and potassium dichromate , which, however, have to be added in a proportion of 1 to 2% by mass (percent by mass). The effect can also be achieved with a significantly smaller amount of 0.1% by mass of potassium hexacyanidoferrate (II) K 4 [Fe (CN) 6 ] .3H 2 O.
With the hygroscopic nature of ammonium nitrate is a strong melting point lowering connected: Even a water absorption of only 1 % by mass lowers the melting point of the salt at about 156 ° C. Conversely, the phase diagram shows a eutectic with a melting point of −16.9 ° C for an ammonium nitrate content of 42% by mass. - the ammonium nitrate, which is very easily soluble in water, causes a lowering of the melting point of the water up to a content of 42% by mass, whereupon its application u. a. based in freezing mixtures . At higher mass fractions , the ammonium nitrate is present in two phases, on the one hand the aqueous solution, on the other hand the salt itself as solid sediment (at 20 ° C from approx. 65% by mass , at 100 ° C from approx. 91.5% by mass ) :
At high pressure, the water solubility drops drastically: at normal pressure and 25 ° C, the mixture still contains 67.6% by mass of ammonium nitrate, up to a pressure of 12 kbar this proportion drops to only 25.4% by mass. At a pressure of 12.1 kbar and a content of 25.3% by mass, the phase boundary lines intersect between the single-phase solution and the two-phase mixture of solution and ice or of solution and ammonium nitrate as polymorph IV. Above this pressure there is a two-phase mixture of ice and solid ammonium nitrate and no solution can exist anymore.
The density of pure ammonium nitrate is 1.725 g · cm −3 . In aqueous solution the density increases with increasing concentration and decreases with increasing temperature.
Density of aqueous ammonium nitrate solutions at different temperatures Salary (Ma%) 20% 30% 40% 50% 60% 70% 80% 90% 94% 20 ° C 1.0830 1.1275 1.1750 1.2250 1.2785 40 ° C 1.0725 1.1160 1.1630 1.2130 1.2660 1.3220 60 ° C 1.0620 1.1045 1.1510 1.2005 1.2525 1.3090 1.3685 80 ° C 1.0550 1.0935 1.1390 1.1875 1.2395 1.2960 1.3550 100 ° C 1.0410 1.0820 1.1270 1.1745 1.2265 1.2825 1.3420 1.4075 120 ° C 1.3285 1.3930 1.4210 140 ° C 1.3785 1.4065 160 ° C 1.3940
In methanol , a 20% solution results at 30 ° C and a 40% solution at 60 ° C. The solubility in ethanol is much lower. Only a 4% solution can be obtained here at 20 ° C.
Chemical properties
When heated (T> 170 ° C) ammonium nitrate breaks down according to the equation
in water and nitrous oxide . Due to a strong initial spark , it disintegrates as follows:
The nitrogen atom of the nitrate ion NO 3 - ( oxidation level + V) oxidizes the nitrogen atom of the ammonium ion NH 4 + (oxidation level −III), so that in the end, in the N 2 molecule, both nitrogen atoms are at the same oxidation level (here 0). Reactions of this kind, in which atoms oxidize other atoms of the same element and are themselves reduced, so that in the end all are at the same oxidation level, are called comproportionations .
The explosive transition from solid (NH 4 NO 3 ) to exclusively gaseous products (H 2 O, N 2 and O 2 ) of this reaction explains the high explosive power of ammonium nitrate: At around 980 l / kg, it has one of the highest specific vapor volumes , and If you also factor in its density, the ratio is even higher (vapor volume / explosives volume). Other important explosion indicators are:
- Heat of explosion : 2625 kJ kg −1
- Detonation velocity : 2500 m · s −1 at a density of 1.4 g · cm −3
- Lead block expansion : 180 cm 3 / 10g
- Impact sensitivity : 49 Nm
- Sensitivity to friction : No reaction up to 353 N pin load
- Steel sleeve test : limit diameter 1.0 mm
By reacting with concentrated sulfuric acid and subsequent distillation , the nitric acid can be recovered, which is the starting substance for the production of many explosives:
use
Ammonium nitrate is the main component of many fertilizers ( ammonium nitrate-urea solution , complex fertilizer ("blue grain"), calcium ammonium nitrate (Nitramoncal, brand name of Chemie Linz, internal NAC )).
It is also used for explosives . Ammonium nitrate is, for example, in the disintegrants ANC , Donarit and Kinepak included.
Ammonium nitrate was also used at times as a propellant for airbags in motor vehicles. However, it proved to be insufficiently long-term stable, especially under the influence of high ambient temperature and humidity, and was replaced by other blowing agents. In the USA alone, over 40 million vehicles had to be recalled because at least one airbag contained a gas generator with ammonium nitrate.
Although it is considered oxidizing and can explode when heated, ammonium nitrate is not one of the actually explosive substances within the meaning of the Explosives Act . Nonetheless, the use of it in the Federal Republic of Germany is regulated by the Explosives Act, and so ammonium nitrate may only be used in fertilizers mixed with harmless substances such as lime (KAS27) because of its potential danger. Typical concentrations at the manufacturer Chemie Linz were formerly 26 and 28% N (nitrogen content). Today, 27% N is common, which means about 70% NH 4 NO 3 and the remainder lime and some oil to prevent the balls from sticking together. Higher nitrogen contents of up to 46% can be achieved with urea , its amide nitrogen is available more slowly.
Disasters
Ammonium nitrate is the cause of numerous explosion disasters :
- Explosion of the ammonia plant of BASF in Oppau (now a district of Ludwigshafen on the Rhine ) on 21 September 1921: solidified ammonium sulfate nitrate -Dünger was there usually fanned before discharging means of dynamite. Due to a change in the production process, there was presumably a local accumulation of ammonium nitrate in the product. The explosions triggered two explosions in quick succession, in which around 400 of a total of 4500 tons of fertilizer detonated in a silo, causing one of the greatest damage in history: 559 people were killed, injured in 1977 and a large part of the factory and the surrounding area Buildings were destroyed. The explosion could be heard as far as Munich, 300 kilometers away.
- In the Texas City explosion on April 16, 1947, the two freighters Grandcamp ( France ) and Highflyer (USA) loaded with approx. 2300 tons of ammonium nitrate exploded in the port of Texas City in the United States . There were 500 to 600 dead, over 100 missing, 8,000 injured, hundreds homeless and $ 65 million in damage.
- On July 28, 1947, the freighter Ocean Liberty ( Norway ) loaded with ammonium nitrate exploded in the port of Brest (France ). There were 26 dead and over 100 injured.
- On 9 January 1963, arrived in Oulu ( Finland ) to an explosion in a nitrogen factory, died when 10 employees.
- In the April 19, 1995 bombing of the Murrah Federal Building in Oklahoma City in the United States, right-wing terrorist Timothy McVeigh murdered 168 people, including 19 children in a kindergarten, and injured more than 800 others by hitting one of his car bombs self-made, 2.4-tonne explosive device made of ammonium nitrate and nitromethane detonated, causing an eight-story administrative building to collapse. Over 300 other buildings were damaged.
- Exactly 80 years after the explosion at the ammonia plant in Oppau (see above), 31 people died on September 21, 2001 in an ammonium nitrate explosion in the AZF fertilizer factory in Toulouse, France. There were also thousands of injuries and huge damage to property.
- In the Ryongchŏn railway accident on April 22, 2004, a train wagon loaded with ammonium nitrate exploded in Ryongchŏn , North Korea, killing at least 161 people. An estimated 1,300 people were injured and 8,000 homes were destroyed or damaged.
- On July 22, 2011, the right-wing extremist assassin Anders Behring Breivik detonated a car bomb based on 950 kilograms of ANFO (ammonium nitrate and diesel oil ) in Norway in the Oslo government district as part of his series of attacks . 8 people were killed and 10 others were injured.
- At least 14 people died and 180 others were injured in a fire and a violent explosion at the West Fertilizer Company in Texas on April 17, 2013.
- On August 12, 2015, hundreds of people were killed or injured in an explosion in Tianjin, China . According to the British newspaper The Guardian , 800 tons of ammonium nitrate were stored on site, along with numerous other substances. This amount is suitable to explain the 100 m wide crater that the explosion left behind. It is not known which substance was the main contributor to the destruction.
- On August 4, 2020, according to the Lebanese Prime Minister, an amount of approx. 2,750 tons of ammonium nitrate destroyed the port of Beirut and large parts of the area in a massive explosion . The explosion was so violent that it could be heard hundreds of kilometers away.
See also
Individual evidence
- ↑ a b c d e f Entry on ammonium nitrate in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ^ A b c L. H. Adams, RE Gibson: Equilibrium in binary systems under pressure. III. The influence of pressure on the solubility of ammonium nitrate in water at 25 ° . In: J. Am. Chem. Soc. tape 54 , no. 12 , 1932, p. 4520–4537 , doi : 10.1021 / ja01351a008 .
- ↑ a b c d e f g h i j k l Karl-Heinz Zapp, Karl-Heinz Wostbrock, Manfred Schäfer, Kimihiko Sato, Herbert Seiter, Werner Zwick, Ruthild Creutziger, Herbert Head: Ammonium Compounds . In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim 2005, ISBN 978-3-527-30673-2 , Ammonium Compounds, doi : 10.1002 / 14356007.a02_243 .
- ↑ Dimethyl Sulfoxide (DMSO) Solubility Data . In: Gaylord Chemical Company, LLC (Ed.): Bulletin 102 . June 2014, p. 15 ( gaylordchemical.com [PDF; accessed August 6, 2020]).
- ↑ Frank-Michael Becker u. a: Formula collection . 3. Edition. Paetec, Berlin 2003, ISBN 3-89818-700-4 , p. 116 .
- ^ Vaclav Smil: Creating the Twentieth Century: Technical Innovations of 1867-1914 and Their Lasting Impact . Oxford University Press, 2005, ISBN 978-0-19-803774-3 , pp. 185 ( books.google.de ).
- ^ A b c Jacqueline Akhavan: The Chemistry of Explosives . Royal Society of Chemistry, 2011, ISBN 978-1-84973-330-4 , pp. 5 ( limited preview in Google Book search).
- ↑ Tor E. Kristensen: A factual clarification and chemical-technical reassessment of the 1921 Oppau explosion disaster the unforeseen explosivity of porous ammonium sulfate nitrate fertilizer. (PDF; 1.6 MB) 16/01508. In: FFI-RAPPORT. Norwegian Defense Research Establishment / Forsvarets forskningsinstitutt, October 4, 2016, p. 20 , accessed on January 1, 2020 .
- ↑ George Stanley Scott, RL Grant: Ammonium Nitrate - Its Properties and Fire and Explosion Hazards . US Department of the Interior, Bureau of Mines, 1948, p. 23 ( limited preview in Google Book search).
- ^ A b c d Rudolf Meyer, Josef Koehler, Axel Homburg, Rudolf Meyer, Axel Homburg ,: Explosivstoffe . 10., completely revised. Edition. Wiley-VCH, Weinheim 2008, ISBN 978-3-527-62341-9 .
- ↑ C. Oommen, SR Jain: Phase Modification of Ammonium Nitrate by Potassium Salts . In: Journal of Thermal Analysis and Calorimetry . tape 55 , no. 3 , 1999, p. 903-918 , doi : 10.1023 / A: 1010146203523 .
- ↑ Anuj A. Vargeese, Satyawati S. Joshi, VN Krishnamurthy: Use of potassium ferrocyanide as habit modifier in the size reduction and phase modification of ammonium nitrate crystals in slurries . In: Journal of Hazardous Materials . tape 180 , no. 1 , 2010, p. 583-589 , doi : 10.1016 / j.jhazmat.2010.04.073 .
- ↑ Anuj A. Vargeese, Krishnamurthi Muralidharan, VN Krishnamurthy: Thermal stability of habit modified ammonium nitrate: Insights from isoconversional kinetic analysis . In: Thermochimica Acta . tape 524 , no. 1 , 2011, p. 165–169 , doi : 10.1016 / j.tca.2011.07.009 .
- ↑ Bernd Engels, Reinhold Fink, Tanja Schirmeister, Carsten Schmuck: Chemistry for medical professionals . 1st edition. Addison-Wesley in Pearson Education Germany, Munich 2008, ISBN 978-3-8273-7286-4 .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 65.
- ^ Neil Gough, Jonathan Soble, Hiroko Tabuchi: Defective Takata Airbag Grows Into Global Problem for Manufacturer. In: New York Times online. November 18, 2014, accessed April 14, 2019 .
- ↑ Takata Airbag Recall: Everything You Need to Know. In: Consumer Reports. March 29, 2013, accessed April 16, 2019 .
- ^ Hans Schuh :: The riddle of Toulouse . In: The time . No. 41 , 2001, p. 39 ( zeit.de - Review of ammonium nitrate disasters.).
- ^ Christian Haller: The explosion accident in BASF from September 21, 1921. Disaster perception and processing in the press, politics and experts . In: Journal for the history of the Upper Rhine . No. 161 , 2013, p. 327-328 .
- ^ RM Goody: The Physics of the Stratosphere . Cambridge University Press, 2014, ISBN 978-1-107-69606-8 , pp. 32 ( full text in Google Book Search).
- ^ Aftermath. ( Memento from November 26, 2015 in the Internet Archive ) In: The 1947 Texas City Disaster. Moore Memorial Public Library, April 2, 2007.
- ↑ Emina Mamaca include: Review of Chemical Spills at Sea and lessons learned. 2009, p. 17 (Interspill Conference of the Center of Documentation, Research and Experimentation on Accidental Water Pollution ).
- ↑ Sébastien Panou: July 28, 1947, l'Ocean Liberty explose. In: maville.com. brest.maville.com, July 28, 2007, accessed August 6, 2020 (French).
- ↑ Kohtalokkaasta tehdasräjähdyksestä 55 vuotta - koko Oulun kaupunki vavahteli ja uhreja on vieläkin kateissa. Retrieved August 8, 2020 (Finnish).
- ↑ Kun Typpi räjähti, koko Oulu heräsi - oululaisten mieleen syöpyneen Typpi Oy: n räjähdyksen aiheutti sama ammoniumnitraatti kuin Beirutin räjähdyksen. Retrieved August 8, 2020 (Finnish).
- ↑ Deadly Texas blast 'like tornado' . In: BBC News . BBC News , April 18, 2013 ( bbc.com ).
- ↑ Fertilizer plant in Texas: explosion leaves a picture of devastation. In: orf.at. news.ORF.at , April 13, 2013, accessed on August 6, 2020 .
- ^ Fergus Ryan: Tianjin explosions: warehouse 'handled toxic chemicals without license' - reports . In: The Guardian . 2015 ( theguardian.com ).
- ↑ Beirut: Where does the dangerous ammonium nitrate come from? In: tagesschau.de. Retrieved August 6, 2020 .
literature
- Committee for Hazardous Substances (Ed.): Technical rule for hazardous substances. TRGS 511 ammonium nitrate . (PDF; 431 kB). November 2008
- Richard Escales: Ammonite Nitrous Explosives . Books on Demand, Norderstedt 2002, ISBN 3-8311-3563-0 (reprint of the 1909 edition)
- Erica Lotspeich, Vilem Petr: The Characterization of Ammonium Nitrate MiniPrills , Chapter 45 in Dynamic Behavior of Materials, Volume 1: Proceedings of the 2014 Annual Conference on Experimental and Applied Mechanics, Springer, 2014, pages 319 ff. ( Googlebooks )
- K. Hahnefeld, R. Gill, G. Buske: Influences on the detonation ability of ammonium nitrate . Wirtschaftsverlag NW, Bremerhaven 1983, ISBN 3-88314-308-1
- W. Pittman, Zhe Han, B. Harding, C. Rosas, Jiaojun Jiang, A. Pineda, MS Mannan: Lessons to be learned from an analysis of ammonium nitrate disasters in the last 100 years . In: J. Hazard. Mat. 280, 2014, pp. 472-477, doi: 10.1016 / j.jhazmat.2014.08.037









