Paralstonite
| Paralstonite | |
|---|---|
| General and classification | |
| other names |
|
| chemical formula | BaCa [CO 3 ] 2 |
|
Mineral class (and possibly department) |
Carbonates and nitrates - carbonates without additional anions; without H 2 O |
|
System no. to Strunz and to Dana |
5.AB.40 ( 8th edition : V / B.04-70 ("Lapis system")) 02/14/02/02 |
| Similar minerals | Alstonite; Alstonite and barytocalcite are also chemically identical |
| Crystallographic Data | |
| Crystal system | trigonal |
| Crystal class ; symbol | trigonal trapezoidal; 32 |
| Space group | P 321 (No. 150) |
| Lattice parameters | a = 8.692 Å ; c = 6.148 Å |
| Formula units | Z = 3 |
| Frequent crystal faces | {22 4 1} |
| Twinning | Yes |
| Physical Properties | |
| Mohs hardness | 4-4.5 |
| Density (g / cm 3 ) | 3.60 (measured); 3.62 (calculated) |
| Cleavage | not specified |
| Break ; Tenacity | uneven; brittle |
| colour | colorless, smoky white, pale gray, in aggregates gray-white; colorless in transmitted light |
| Line color | White |
| transparency | translucent to translucent |
| shine | Glass gloss |
| Crystal optics | |
| Refractive indices |
n ω = 1.672 n ε = 1.527 |
| Birefringence | δ = 0.145 |
| Optical character | uniaxial negative |
| Other properties | |
| Chemical behavior | vigorous foaming in dilute HCl |
| Special features | fluoresces pale to light orange under long-wave UV light, no cathodoluminescence |
Paralstonite is a very rarely occurring mineral from the mineral class "carbonates and nitrates" (formerly carbonates, nitrates and borates ). It crystallizes in the trigonal crystal system with the idealized chemical composition Baca [CO 3 ] 2 , and is thus chemically seen a barium - calcium - carbonate .
At the type locality, paralstonite develops crusts of idiomorphic , pyramidal crystals up to 1 mm in size, which are rarely terminated on both sides and are thus dipyramidal. There are also powdery and fibrous mineral aggregates .
The type locality of the paralstonite is the "Minerva No. 1 Mine “(Ozark-Mahoning No. 1 Mine) ( coordinates of Minerva No. 1 Mine ) at Cave-in-Rock near Rosiclare in Hardin Co. , Illinois , United States .
Etymology and history
While working on his dissertation on the mineral alstonite , the Canadian mineralogist Andrew C. Roberts noticed a mineral on steps from the Cave-in-Rock-District, Illinois, USA, which was chemically indistinguishable from alstonite and had an X-ray diffraction pattern. which could not be assigned to a known phase. Roberts referred to the phase as "unnamed barium calcium carbonate" (unnamed barium calcium carbonate)
After Roberts had determined the required physical and optical properties and the chemical composition as well as the crystal structure by Herta Silvia Effenberger from the "Institute for Mineralogy and Crystallography" of the University of Vienna in Austria , the mineral was submitted to the International Mineralogical Association (IMA), which it Recognized as a new mineral in 1979 under the provisional designation IMA 1979-015. In the same year, Andrew C. Roberts published the first scientific description of this mineral in the Canadian science magazine " Geological Survey of Canada Paper ". He named the mineral because of its morphological, chemical and crystallographic similarity with alstonite and after the Greek word παρά [para] for "related to" - put together "related to alstonite" - as paralstonite ( English paralstonite ).
The type material (holotype) for paralstonite is stored under catalog number 13380 in the collection of the Geological Survey of Canada in Ottawa , Canada . Another type of material is in the collections of the Royal Ontario Museum , Toronto , Canada, and the Smithsonian Institution belonging to National Museum of Natural History , Washington, DC , USA (catalog number 145915).
classification
Since the paralstonite was only recognized as an independent mineral by the International Mineralogical Association (IMA) in 1979 and the discovery was published in the same year, it is not listed in the 8th edition of the Strunz mineral systematics, which has been obsolete since 1977 .
In the last revised and updated Lapis mineral directory in 2018 , which is still based on this outdated system of Karl Hugo Strunz out of consideration for private collectors and institutional collections , the mineral was given the system and mineral number. V / B.04-70 . In the "Lapis system" this corresponds to the section "Anhydrous carbonates [CO 3 ] 2− , without foreign anions ", where paralstonite together with alstonite, aragonite , barytocalcite , cerussite , olekminsite , strontianite and witherite form the "aragonite group" (V / B.04) forms.
The 9th edition of Strunz's mineral systematics, valid since 2001 and updated by the International Mineralogical Association (IMA) until 2009, assigns paralstonite to the “carbonates and nitrates” class, which has been reduced by the borates, and to the “carbonates without additional” class Anions; without H 2 O “. This is further subdivided according to the group affiliation of the cations involved , so that the mineral can be found according to its composition in the sub-section "alkaline earth (and other M 2+ ) carbonates", where, together with Olekminskit, the unnamed group with the system no . 5.AB.40 forms.
The systematics of minerals according to Dana , which is mainly used in the English-speaking world , assigns the paralstonite to the common class of "carbonates, nitrates and borates" and there in the department of "anhydrous carbonates", like the outdated Strunz system. Here he is together with Norsethit and Olekminskit in the " Norsethitgruppe " with the system no. 02/14/02 within the sub-section "Anhydrous carbonates with the formula A + B 2+ (CO 3 ) 2 ".
Chemism
Microprobe analyzes on paralstonite from the type locality yielded 18.8% CaO; 45.6% BaO; 5.2% SrO; and 30.0% CO 2 (calculated from stoichiometry); Total 99.6%. Based on six oxygen atoms per formula unit, the empirical formula Ba 0.872 Ca 0.983 Sr 0.147 (CO 3 ) 2.00 is calculated, which can be idealized to BaCa (CO 3 ) 2 . This idealized formula requires 51.55% BaO; 18.86 CaO and 29.59% CO 2 . The idealized formula corresponds to the official formula of the IMA for paralstonite, in the formula according to Strunz, BaCa [CO 3 ] 2 , the anion group is given in square brackets.
Microprobe analyzes on paralstonite from the alkali rock massif Kedrovyi in the Murunskii massif in the Aldan highlands , Republic of Sakha (Yakutia) , Federal District Far East , Russia , yielded 19.42% CaO; 51.23% BaO; 0.50% SrO; and 30.1% CO 2 (calculated from stoichiometry); Total 101.25%.
The only combination of elements Ba – Ca – C – O or the chemical composition BaCa (CO 3 ) 2 have, among the currently known minerals, not only trigonal paralstonite but also triclinic alstonite and monoclinic barytocalcite. BaCa (CO 3 ) 2 is therefore polymorphic . In addition to the three minerals with the chemical composition BaCa (CO 3 ) 2 , there is also a new synthetic phase which, like barytocalcite , crystallizes monoclinically, but in the space group C 2 (no. 5) .
Chemically similar are z. B. Podlesnoite , BaCa 2 (CO 3 ) 2 F 2 , benstonite , Ba 6 Ca 6 Mg (CO 3 ) 13 , and carbocernaite , (Ca, Na) (Sr, Ce, Ba) (CO 3 ) 2 . Chemically Paralstonit can be used as the calcium-dominant analogue of the Mg-dominated Norsethit , BaMg (CO 3 ) 2 , to be construed.
Paralstonite forms a mixed crystal row with olekminsite, Sr 2 (CO 3 ) or Sr (Sr, Ca, Ba) (CO 3 ), but this is probably incomplete and therefore has miscibility gaps .
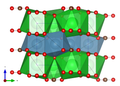
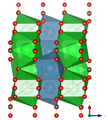
Crystal structure
Paralstonite crystallizes in the trigonal crystal system in the space group P 321 (space group no. 150) with the lattice parameters a = 8.692 Å and c = 6.148 Å as well as three formula units per unit cell .
The crystal structure of the paralstonite was determined in 1980 by Herta Sylvia Effenberger - the spatial representation of the paralstonite structure opposite was drawn based on her data.
| Crystal structure of paralstonite. The blue outline shows the unit cell. |
|
|
| Color legend: __ Ba __ Ca __ C __ O |
In the crystal structure of paralstonite, Ba 2+ is linked in a 10-coordinated geometry with ten O 2− atoms (Ba [10] ), while Ca is coordinated by eight oxygen atoms (Ca [8] ). Furthermore, there are three crystallographically different, planar (CO 3 ) 2− groups. All units are arranged in an “ABAB…” stacking sequence parallel to (0001).
properties
morphology
At the type locality, paralstonite was found in the form of idiomorphic crystals with a pyramidal habit up to 1 mm in size, which show the trigonal dipyramid II position {22 4 1} as a supporting form . Usually only the faces of one half of the crystal can be seen, and dipyramidal crystals have rarely been observed. The length / width ratio of the crystals is approximately 1: 2. The crystal faces are strongly striped at right angles to the axis of the longitudinal extension (or the c-axis [001]) and are divided parallel to [001] by a medial, irregular, slightly receding suture (seam-like line of fusion). Single crystal recordings show that the mineral is generally twinned.
In the “Dolyhir Quarry”, Wethel , Old Radnor , Powys , Wales , paralstonite overgrows in tiny, “prickly” crystals 0.1 mm in size, harmotome and ewaldite and also cracks in calcite . There are also powdery and fibrous mineral aggregates here . According to X-ray diffractometric analyzes, such inconspicuous, powdery, chalk-white and microcrystalline crusts consist of alstonite and paralstonite. Due to the powdery nature of these aggregates, it is impossible to visually distinguish between the two phases. The no more than 0.1 mm long crystals of paralstonite, which are visible under the scanning electron microscope , are shaped like grains of rice and are generally more steeply terminated than those of alstonite.
Up to 2 mm long, hexagonal crystals on brown calcite from the Cave-in-Rock district were found to be displacement pseudomorphs from strontium-containing barite to paralstonite, whereby the core of these pseudomorphs often still contains the original paralstonite - the displacement process consequently began on the surface of the crystals.
physical and chemical properties
The crystals of Paralstonite are colorless, smoky white or pale gray, in aggregates also gray-white. Their line color , however, is always white. The surfaces of the translucent to transparent crystals show a characteristic glass-like sheen . According to this glass gloss, paralstonite has a medium-high refraction ( n ε = 1.527; n ω = 1.672) and a very high birefringence (δ = 0.145). In transmitted light, the uniaxial negative paralstonite is colorless and shows no pleochroism.
There is no information regarding the cleavage of paralstonite. Due to its brittleness , the mineral breaks like amblygonite , whereby the fracture surfaces are uneven. Paralstonite has a Mohs hardness of 4 to 4.5 and thus belongs to the medium-hard minerals that, like the reference mineral fluorite (hardness 4), are more or less easy scratch with a penknife. The measured density for paralstonite is 3.60 g / cm³, the calculated density is 3.62 g / cm³.
Paralstonite shows variable, pale to light orange fluorescence in long-wave UV light and no cathodoluminescence . The mineral dissolves with vigorous foaming in dilute hydrochloric acid , HCl.
Education and Locations
Paralstonit formed in low-temperature, lead and - zinc -Erze leading buntmetall leading veins . Minerals accompanying paralstonite at its type locality are yellow calcite scalenohedra, massive, spherical barite , gray alstonite, purple fluorite and brown sphalerite . Paralstonite covers the spherical barite in the form of thin layers of colorless, hexagonal dipyramids. In the alkaline rock massif “Kedrovyi”, Russia, paralstonite is accompanied by olekminsite, calcite, barite, ankerite , ankylite (Ce) , narsarsukite , sphalerite and galena . In the “Dolyhir Quarry”, Wales, the paralstonite forms in thin cracks and fissures in sediments and dolerites of the Precambrian “Yat Wood Formation”. Here belong to the companions of paralstonite u. a. Alstonite, Harmotome, Calcite, Ewaldite and Quartz.
As a very rare mineral formation, paralstonite is so far (as of 2019) known from only seven discovery points. The type locality of the paralstonite is the "Cave-in-Rock Mining Sub-District", Hardin Co. , Illinois , USA . The exact location is unknown, but it is very likely to be the “Bethel Level” of the “Minerva No.” belonging to the “Ozark-Mahoning Group”. 1 Mine ”(also known as“ Ozark-Mahoning No. 1 Mine ”). A second location in the USA is the "Annabel Lee Mine" in the "Harris Creek Mining Sub-District", also in Hardin County, Illinois.
Further find points for paralstonite are:
- the "Dolyhir Quarry", Wethel , Old Radnor , Powys , Wales
- the "Seblyavr" massif southwest of Murmansk , Kola Rajon , Murmansk Oblast , Kola Peninsula , Russia
- the alkaline rock massif "Kedrovyi" in the Murunskii massif ( Russian Мурунский массив ) at the confluence of the Tschara and Tokko in the Aldan highlands , Republic of Sakha (Yakutia) , Federal District Far East , Russia
- the Kimberlitschlot Udachnaya pipe ( Russian Трубка Удачная ) (also Udachnaya-Vostochnaya pipe; Udachnaya Pipe) at udachny on the Wiljuiplateau the river Daldyn , Rajon Mirny (Sacha) , Republic of Sakha (Yakutia), Russia
- the vast polygenic REE - iron - niobium -Lagerstätte Bayan Obo mining district of Bayan Obo north of the Borough of Bayan-Obo the prefecture-level city Baotou , Autonomous Region of Inner Mongolia in the People's Republic of China
Locations from Germany , Austria and Switzerland are therefore unknown for paralstonite.
use
Paralstonite is economically insignificant and only a mineral sought after by mineral collectors.
See also
literature
- Andrew C. Roberts: Mineralogical study of an unnamed barium calcium carbonate from the Cave-in-Rock district, Illinois . In: Geological Survey of Canada Paper . 78-1C, 1978, pp. 49–52 (English, rruff.info [PDF; 313 kB ; accessed on October 17, 2019]).
- Andrew C. Roberts: Paralstonite: A new mineral from the Minerva No. 1 mine, Cave-in-Rock, Illinois . In: Geological Survey of Canada Paper . 79-1C, 1979, pp. 99–100 (English, rruff.info [PDF; 126 kB ; accessed on October 17, 2019]).
- Paralstonite . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 ( handbookofmineralogy.org [PDF; 65 kB ; accessed on October 17, 2019]).
Web links
- Mineral Atlas: Paralstonite (Wiki)
- Paralstonite. In: mindat.org. Hudson Institute of Mineralogy, accessed October 17, 2019 .
- David Barthelmy: Paralstonite Mineral Data. In: webmineral.com. Retrieved October 17, 2019 .
- American-Mineralogist-Crystal-Structure-Database - Paralstonite. In: rruff.geo.arizona.edu. Retrieved October 17, 2019 .
Individual evidence
- ↑ a b c Andrew C. Roberts: A mineralogical investigation of alstonite BaCa (CO 3 ) 2 (unpubl. M.Sc. thesis) . Queen's University, Kingston, Ontario, Canada 1976, p. 1–58 (English, researchgate.net [PDF; 4.5 MB ; accessed on October 17, 2019]).
- ↑ a b c d e f g h i Andrew C. Roberts: Mineralogical study of an unnamed barium calcium carbonate from the Cave-in-Rock district, Illinois . In: Geological Survey of Canada Paper . 78-1C, 1978, pp. 49–52 (English, rruff.info [PDF; 313 kB ; accessed on October 17, 2019]).
- ↑ a b Malcolm Back, William D. Birch, Michel Blondieau and others: The New IMA List of Minerals - A Work in Progress - Updated: September 2019. (PDF 2692 kB) In: cnmnc.main.jp. IMA / CNMNC, Marco Pasero, September 2019, accessed October 20, 2019 .
- ↑ a b c d Minerals with Ba, Ca, C, O. In: mindat.org. Hudson Institute of Mineralogy, accessed October 4, 2019 .
- ↑ a b c d e Paralstonite . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 ( handbookofmineralogy.org [PDF; 65 kB ; accessed on October 17, 2019]).
- ↑ a b c d Hugo Strunz , Ernest H. Nickel: Strunz Mineralogical Tables . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 289 .
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak Andrew C. Roberts: Paralstonite: A new mineral from the Minerva No. 1 mine, Cave-in-Rock, Illinois . In: Geological Survey of Canada Paper . 79-1C, 1979, pp. 99–100 (English, rruff.info [PDF; 126 kB ; accessed on October 17, 2019]).
- ↑ Stefan Weiß: The large Lapis mineral directory. All minerals from A - Z and their properties. Status 03/2018 . 7th, completely revised and supplemented edition. Weise, Munich 2018, ISBN 978-3-921656-83-9 .
- ↑ Ernest H. Nickel, Monte C. Nichols: IMA / CNMNC List of Minerals 2009. (PDF 1703 kB) In: cnmnc.main.jp. IMA / CNMNC, January 2009, accessed September 25, 2019 .
- ↑ Dominik Spahr, Lkhamsuren Bayarjargal, Victor Vinograd, Rita Luchitskaia, Victor Milman, Björn Winkler: A new BaCa (CO 3 ) 2 polymorph . In: Acta Crystallographica Section B . tape 75 , no. 3 , 2019, p. 291-300 , doi : 10.1107 / S2052520619003238 (English).
- ↑ a b Paralstonite. In: mindat.org. Hudson Institute of Mineralogy, accessed October 17, 2019 .
- ↑ Herta Sylvia Effenberger: The crystal structure of the mineral paralstonite, BaCa (CO 3 ) 2 . In: New yearbook for mineralogy, monthly books . tape 1980 , 1980, pp. 353-363 .
- ^ Luke LY Chang , Robert Andrew Howie , Jack Zussman : Rock-forming minerals Vol. 5B: Mon-silicates: Sulphates, Carbonates, Phosphates and Halides . 2nd Edition. Longman, London 1996, ISBN 0-582-30093-2 , pp. 263–271 (English, limited preview in Google Book Search - first edition: 1961).
- ↑ a b c d Tom F. Cotterell, David I. Green, Neil Hubbard, John S. Mason, Roy E. Starkey: The mineralogy of Dolyhir quarry, Old Radnor, Powys, Wales . In: UK Journal of Mines & Minerals . tape 32 , 2011, p. 5–61 (English, researchgate.net [PDF; 5.5 MB ; accessed on October 17, 2019]).
- ↑ a b c Paralstonite. In: museum.wales. Amgueddfa Cymru - National Museum Wales, accessed October 17, 2019 .
- ↑ Carl A. Francis, David E. Lange, Lawrence C. Pitman, William J. Croft, Ross C. Lillie: Barite after Paralstonite, a New Pseudomorph from Cave-in-Rock, Illinois . In: The Mineralogical Record . tape 28 , no. 6 , 1997, pp. 443-446 (English).
- ↑ J. Theo Kloprogge, Rob Lavinsky, stretch Young: Photo Atlas of Mineral Pseudomorphism . 1st edition. Elsevier, Amsterdam 2017, ISBN 978-0-12-803674-7 , pp. 80 (English, limited preview in Google Book search).
- ↑ Localities for Paralstonite. In: mindat.org. Hudson Institute of Mineralogy, accessed October 17, 2019 .
- ↑ a b List of locations for paralstonite in the Mineralienatlas and Mindat (accessed on October 4, 2019)
- ^ Alan Goldstein: The Illinois-Kentucky fluorspar district . In: The Mineralogical Record . tape 28 , no. 1 , 1997, p. 3-49 (English).
- ↑ Minerva No. 1 mine. In: mindat.org. Hudson Institute of Mineralogy, accessed October 17, 2019 .
- ↑ Tom F. Cotterell, Allan C. Dean: The first British occurrence of paralstonite at Dolyhir Quarry, Old Radnor, Powys, Wales . In: UK Journal of Mines & Minerals . tape 28 , 2007, pp. 31-35 (English).
- ↑ Natalia V. Sorokhtina, Nikita V. Chukanov, Anatolii V. Voloshin, Yakov A. Pakhomovsky, Alla N. Bogdanova, Mikhail M. Moiseev: Cymrite as an indicator of high barium activity in the formation of hydrothermal rocks related to carbonatites of the Kola Peninsula . In: Geology of Ore Deposits . tape 50 , no. 7 , 2008, p. 620–628 , doi : 10.1134 / s1075701508070131 (English).
- ↑ Ekaterina Reguir: Aspects of the mineralogy of the Murun alakline complex, Yakutia, Russia . Master of Science Thesis. Department of Geology, Lakehead University, Thunder Bay, Ontario, Canada 2001 (English, 193 pp., Https://www.collectionscanada.gc.ca/obj/s4/f2/dsk3/ftp04/MQ60867.pdf collectionscanada.gc. ca [PDF; 12.8 MB ; accessed on October 4, 2019]).
- ↑ AA Konyev, EI Vorobyev, LF Piskunova, ZF Ushchalovskaya, GA Tokhonova: Olekminskite Sr (Sr, Ca, B) (CO 3 ) 2 , a new mineral and the new isomorphous series olekminskite-paralstonite . In: Zapiski Vserossiyskogo Mineralogicheskogo Obshchestva . tape 120 , no. 3 , 1991, pp. 89–96 (Russian, rruff.info [PDF; 1.1 MB ; accessed on October 17, 2019]).
- ^ Igor V. Pekov: Minerals first discovered on the territory of the former Soviet Union . 1st edition. Ocean Pictures, Moscow 1998, ISBN 5-900395-16-2 , pp. 155-156 (English).
- ↑ Victor V. Sharygin, Vadim S. Kamenetsky, Maya B. Kamenetsky: Potassium sulfides in kimberlite-hosted chloride- “nyerereite” and chloride clasts of Udachnaya-East pipe, Yakutia, Russia . In: The Canadian Mineralogist . tape 46 , no. 4 , 2008, p. 1079-1095 , doi : 10.3749 / canmin.46.4.1079 (English, https://rruff-2.geo.arizona.edu/uploads/CM46_1079.pdf rruff.info [PDF; 1.1 MB ; accessed on October 17, 2019]).
- ↑ Zhang Peishan, Yang Zhuming, Tao Kejie, Yang Xueming: Mineralogy and Geology of Rare Earths in China (A series of solid earth sciences research in China) . 1st edition. Science Press, Beijing 1996, ISBN 7-03-004904-7 , pp. 1-209 (English).