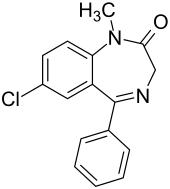
Diazepam
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Diazepam | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 16 H 13 ClN 2 O | ||||||||||||||||||
| Brief description |
pale yellowish solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| Mechanism of action | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 284.74 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
125-126 ° C |
||||||||||||||||||
| pK s value |
3.4 |
||||||||||||||||||
| solubility | |||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Diazepam , originally marketed as Valium , is a psycho-active effective drug from the group of benzodiazepines with a relatively long half-life . It is indicated for the treatment of anxiety , for the therapy of epileptic seizures and for the premedication before surgical and diagnostic interventions. Since long-term therapy with diazepam can lead to psychological and physical dependence , the active ingredient is primarily used in acute therapy - that is, for no longer than four to six weeks. Diazepam is listed on the World Health Organization (WHO) list of Essential Medicines .
Clinical information
application areas
In addition to its use for the symptomatic treatment of acute states of tension, excitement and anxiety , diazepam is used in premedication before surgical and diagnostic interventions. It is also used as a muscle relaxant and as an emergency therapeutic agent for the prophylaxis and anticonvulsive treatment of epileptic grand mal seizures , for the treatment of febrile convulsions that occur in children. Diazepam is only justified as a sleep aid if the diazepam effects during the day are also desired.
Because of the risk of developing addiction, the duration of treatment should be as short as possible. Diazepam should be used with extreme caution in patients with a history of drug, alcohol, or other drug dependence . Diazepam is also used intravenously (iv) as a life-saving antidote for chloroquine poisoning.
Contraindications
Contra-indications for treatment with diazepam are generally respiratory insufficiency (heavy breathing), nocturnal interruption of breathing ( sleep apnea syndrome ), severe hepatic insufficiency (liver disease), myasthenia gravis (pathological muscular weakness ), and a dependency history (pharmaceuticals, alcohol, other drugs) or acute intoxication with alcohol , sleeping pills, painkillers or psychotropic drugs .
Dependency
If taken regularly, diazepam can cause psychological as well as physical dependence over a short period of time. This applies not only to improper use, but also to the therapeutic dose range. In Germany around 1.9 million people are dependent on substances belonging to the benzodiazepine class. Sudden discontinuation of therapy after prolonged use can cause severe withdrawal symptoms .
Use in pregnancy
Heart malformations, cleft lip / palate, and complex other malformations have been reported in some studies . Other studies could not confirm teratogenic effects. There are not enough studies on the later development of the child to be able to make reliable statements about it. There are individual case reports on malformations and intellectual disabilities in prenatally exposed children following overdoses and poisoning. Animal studies have shown evidence of behavioral disorders in the offspring of dams given diazepam.
If diazepam is taken regularly in the 2nd to 3rd trimester or in high doses before or during childbirth, it can cause serious undesirable effects in the newborn.
Interactions with other substances and restrictions on use
Other drugs that affect the brain (for example tranquilizers, sleeping pills, anti-depression drugs, various pain relievers, anti-seizure drugs (anti-epileptic drugs) or muscle relaxants ), as well as certain drugs for gastric ulcers , tuberculosis , fungal diseases , asthma or for alcohol cessation and diazepam can be used may influence each other. Diazepam is broken down by the cytochrome P450 enzyme system (including CYP3A4). Inhibitors of this enzyme system (e.g. cimetidine ) lead to a slower breakdown of diazepam combined with its prolonged or intensified effect. Diazepam also increases the effects of other muscle relaxants as well as the effects of nitrous oxide and analgesics . The use of diazepam and omeprazole , cimetidine, ketoconazole , fluvoxamine , fluoxetine should be avoided as these substances slow down the breakdown of diazepam.
When combining diazepam with other centrally acting substances such as alcohol , neuroleptics , anxiolytics / sedatives , antidepressants , hypnotics , anticonvulsants , narcotic analgesics , anesthetics and sedating antihistamines , it must be taken into account that their effects can mutually reinforce one another.
unwanted effects
Diazepam leads to a reduction in skeletal muscle tone and drowsiness, thereby impairing the ability to react for a long time.
Withdrawal symptoms can be: anxiety, hallucinations , seizures, psychoses , hypersensitivity to noise and light, optical distortion of perception , excessive feeling. Studies give a detailed overview.
Possible side effects with Diazepam are:
tiredness, strong daytime sedation, drowsiness, drowsiness, fatigue, dizziness, headache, ataxia , prolonged reaction time, confusion, anterograde amnesia. Overhang effects (concentration disorders, residual tiredness), impairment of the ability to react.
With high doses and especially with long-term treatment with diazepam:
articulation disorders , movement insecurity and gait insecurity with increased frequency of falls, double vision, nystagmus , states of excitement, anxiety (reversed effect), increased muscle cramps, difficulty falling asleep and staying asleep, fits of anger, hallucinations, suicidality. Experience of derealization and depersonalization as well as cold feelings and weakness in criticism are typical for long-term use of diazepam.
Symptoms of a history of addiction:
Personality change: indifference, loss of drive, dysphoric mood , indifferent to euphoric basic mood (meaningless feeling of happiness), lack of ability to deal with stress and conflict, lack of advance planning (“living into the day”), restricted attention, impaired concentration, general emotional and physical (Psychomotor) slowdown, reaction time slowdown with potentially dangerous consequences in traffic, work and household, forgetfulness (memory gaps): memory loss with regard to the inclusion of new information in the long-term memory, but not with regard to the ability to remember earlier (before the abuse) learned content, organic brain psychosyndrome or Drug-related dementia in older people, whose metabolism can only break down long-acting benzodiazepines and their active intermediates very slowly, lack of resilience with decreased performance, dysphoric Depressive mood, changing moods, loss of control with irritability and aggressive breakthroughs, sometimes downright hostile behavior, inner restlessness, nervousness, agility, inexplicable and indeterminate anxiety states: Tranquilizers reinforce the originally existing fear in the long term (after four months at the latest, there are no fear-reducing effects at all ), increasing fearfulness (of situations, people, things), flight from reality (avoidance behavior), occasional disorientation (local, temporal, personal, in extreme cases confused states), inexplicable clouding of consciousness, delirium-like states, delusional reactions with deceptive perception.
It is known that the use of diazepam can lead to paradoxical reactions such as restlessness, agitation, irritability, aggressiveness, delusions, outbursts of anger, nightmares, hallucinations, psychoses, abnormal behavior and other behavioral disorders. Rebound symptoms may occur when stopping diazepam , i.e. H. the original symptoms that led to treatment with diazepam may become more pronounced.
In the event of an overdose, dizziness and short-term amnesia as well as severe coordination disorders and lisp can occur. In addition, diazepam in high overdoses can cause respiratory depression up to respiratory arrest . Among other things, this leads to a drop in blood pressure and even cardiovascular arrest. The specific antagonist flumazenil can be used as an antidote for poisoning with benzodiazepines .
The half-life is between 48 and 72 hours, i.e. i.e. after this time, half of the original dose is still effective in the body. With repeated intake on several subsequent days, the substance accumulates in the body.
Pharmacological properties
Diazepam has an anxiolytic (anxiolytic), anticonvulsant (anti-epileptic), muscle-relaxing (muscle-relaxing) and sedative (calming) effect.
Mechanism of action
Diazepam acts as an allosteric modulator of the GABA A receptor and increases the inhibitory effect of the neurotransmitter γ-aminobutyric acid (GABA). As an agonist , diazepam binds to the benzodiazepine binding site of this receptor (a chloride ion channel ) and thus causes its conformational change; this increases the receptor sensitivity to GABA. An increased GABA activity results in an increased opening rate at the chloride channel and thus in an increased influx of chloride ions into the cell. The increase in the intracellular chloride concentration leads to a reduced excitability of the cell due to hyperpolarization .
Pharmacokinetics
Diazepam is almost completely absorbed after ingestion . The metabolism mainly comprises the steps N - demethylation and hydroxylation and provides the active breakdown substances desmethyldiazepam, temazepam and oxazepam .
It has a long half-life (20 to 100 hours) and shows a rapid onset of action due to its high lipid solubility and therefore good passage through the blood-brain barrier , but has only a short duration of action due to the rapid redistribution from the brain ( iv "bolus") Diazepam only 10 to 20 minutes). Its breakdown products are also pharmacologically active (half-life of 50 to 80 hours). The breakdown of diazepam is age-dependent. The half-life is around 30 hours in middle-aged adults, while it is around 81 hours in 60 to 90-year-olds.
chemistry
Diazepam is an N -methylated benzodiazepine and is characterized by a lactam structure. Diazepam was first synthesized in the 1950s by Leo Sternbach starting from chlordiazepoxide . Alternatively, Leo Sternbach published a synthetic route starting from p -chloroaniline via 2-amino-5-chlorobenzophenone and glycine ethyl ester hydrochloride.
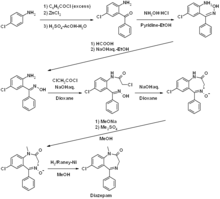
Development and marketing
Diazepam was developed by Leo Sternbach and first brought onto the market in 1963 by F. Hoffmann-La Roche under the trade name Valium . After chlordiazepoxide ( Librium ) in 1960, it was the second benzodiazepine. In Germany, diazepam was still the most frequently prescribed benzodiazepine in 2005.
Natural occurrence
Diazepam (along with temazepam ) is formed in small amounts in potato tops. However, the amounts (60-450 ng / g) are too low to be pharmacologically significant.
Trade names
Faustan (formerly D), Gewacalm (A), Paceum (CH), Valium (CH, USA), Psychopax (A, CH), Relanium (PL), Stesolid (rectal tubes) (D, A, CH), approval for " Valiquid "and the original preparation" Valium "expired in D 2015. Only generic drugs containing diazepam are available in Germany.
See also
literature
- LH Sternbach: The Benzodiazepine Story. In: J. Med. Chem. Volume 22, 1979, pp. 1-7. PMID 34039
- Product information for Valium, Roche Pharmaceuticals
- Borwin Bandelow among others: Handbook of Drug Therapy, Volume 1, Psychopharmaka. 2nd Edition. Enke, 2004, ISBN 3-13-113041-5 .
- Otto Benkert among others: Compendium of Psychiatric Pharmacotherapy - with 59 tables. 5th, completely revised. and exp. Edition. Springer, Berlin / Heidelberg 2004, ISBN 3-540-21893-9 .
Web links
Individual evidence
- ↑ a b entry on diazepam. In: Römpp Online . Georg Thieme Verlag, accessed on June 1, 2014.
- ↑ a b c d e f g Diazepam data sheet from Sigma-Aldrich , accessed on March 24, 2011 ( PDF ).
- ↑ Martin Wehling (Ed.): Clinical Pharmacology. Georg Thieme Verlag, Stuttgart 2005, p. 487.
- ↑ a b c Technical information of the Swiss Medicines Compendium: Valium, information as of February 2006.
- ↑ Antidottarium the Red List
- ↑ Chloroquine. ( Memento from September 18, 2012 in the web archive archive.today ) at Toxinfo.org, Toxicological Department of the II. Medical Clinic of the Technical University of Munich
- ^ Pharmawiki: Diazepam .
- ^ Technical information Diazep-CT 10 mg tablets, AbZ-Pharma GmbH (Ulm), as of October 2013.
- ↑ embryotox.de: Pregnancy, breastfeeding and psychological disorders ( Memento of the original from March 10, 2013 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. As of April 21, 2008.
- ↑ Diazepam ( Memento of the original from July 9, 2018 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. on embryotox.de, as of November 10, 2017. Accessed July 8, 2018.
- ↑ Specialist information of the Swiss Medicines Compendium: Paceum, information as of February 2006.
- ↑ CH Ashton: Benzodiazepines: Mode of action and therapeutic withdrawal. Retrieved June 5, 2013.
- ↑ A. Ballokova, NM Peel, D. Fialova, IA Scott, LC Gray, RE Hubbard: Use of benzodiazepines and association with appropriate in older people admitted to hospital: a prospective cohort study. In: Drugs Aging. 31 (4), Apr 2014, pp. 299-310. PMID 24566878
- ↑ LH Sternbach, S. Kaiser, E. Reeder: Quinazoline 3-Oxide Structure of Compounds Previously Described in the Literature as 3.1.4-Benzoxadiazepines. In: J. Am. Chem. Soc . 82, 1960, pp. 475-480; doi: 10.1021 / ja01487a058 .
- ↑ LH Sternbach , E. Reeder, O. Keller, W. Metlesics: Quinazolines and 1,4-Benzodiazepines. III. Substituted 2-Amino-5-phenyl-3H-1,4-benzodiazepine 4-Oxides. In: J. Org. Chem. 26, 1961, pp. 4488-4497; doi: 10.1021 / jo01069a069 .
- ↑ ePsy.de Psychotropic Drugs in Practice 2005.
- ↑ Dominique Kavvadias: Ligands of the benzodiazepine receptor: Studies on benzodiazepines in plant tissues and on hispidulin. (PDF; 1.8 MB) Dissertation. Julius Maximilians University of Würzburg , 2003, p. 5.