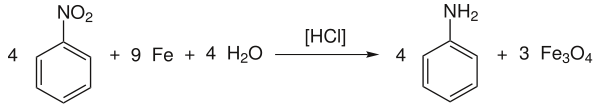aniline
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | aniline | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 6 H 7 N | |||||||||||||||||||||
| Brief description |
oily, colorless to brown liquid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 93.13 g mol −1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
1.02 g cm −3 |
|||||||||||||||||||||
| Melting point |
−6 ° C |
|||||||||||||||||||||
| boiling point |
184 ° C |
|||||||||||||||||||||
| Vapor pressure |
0.681 hPa (20 ° C) |
|||||||||||||||||||||
| solubility |
poor in water (36 g l −1 at 20 ° C) |
|||||||||||||||||||||
| Refractive index |
1.5863 (20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| MAK |
|
|||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
Aniline (from Spanish or Arabic : an-nil = blue = indigo color ) or benzenamine is a clear, colorless to slightly yellowish, oily liquid with a peculiar smell that quickly turns reddish-brown in the air. It is a benzene ring with an amino group (–NH 2 ) and thus an aromatic amine . When mixed with acids , it forms aniline salts . The basic effect of aniline is weakened by the mesomeric effect , as this reduces the electron density of the amino group.
nomenclature
The IUPAC systematic name is Benzenamine. However, the now preferred IUPAC name is aniline.
history
Aniline was first produced by Otto Unverdorben in 1826 by distilling lime from indigo . He called the oil obtained crystallin (a characteristic property is the formation of crystallizable salts with acids). In 1834 Friedlieb Ferdinand Runge isolated aniline for the first time from what was long the most important source, coal tar , and named it kyanol (blue oil, after the behavior of the substance towards chlorinated lime solution). Carl Julius Fritzsche had received the aniline from the distillation of anthranilic acid in 1840 ; he was also able to prove the identity of the product Zinin presented. Zinin obtained aniline (which he called benzidam ) from nitrobenzene by reducing it with hydrogen sulfide. AW Hofmann showed that these compounds are identical; he was also able to reduce the nitrobenzene to aniline with a significantly improved process (zinc + acid).
Aniline has been used by the Badische Anilin- und Soda-Fabrik ( BASF ) since 1897 for the synthesis of the dye indigo, which was previously only obtained from vegetable raw materials ( Heumann synthesis ). Aniline had already been produced on a large scale beforehand, for example by Agfa ( Actien-Gesellschaft für Anilin-Fabrication ) from 1873 onwards. A well-known use of the dye was aniline leather . Aniline was also used in printing technology . a. who got flexo nicknamed flexographic still used today, as could be produced only by the aniline good print quality.
Manufacturing
Industrially aniline by hydrogenation of nitrobenzene in the gas phase at 270 ° C and 1.25 bar at a copper catalyst on silica prepared. A fluidized bed reactor is used for this.
In addition, hydrogenation over a nickel sulfide catalyst on aluminum oxide in a fixed bed reactor is possible.
The process by which aniline was obtained by reducing nitrobenzene with iron in the presence of hydrochloric acid ( Béchamp reduction ) is out of date:
It is then neutralized with quicklime (CaO) and the aniline is distilled off together with the water. The iron (II, III) oxide formed as a by-product can be used as a pigment .
There are other processes, for example the ammonolysis of chlorobenzene or phenol :
use
In the chemical industry, it is primarily used as a raw material for the synthesis of colors and synthetic fibers, but also for the production of synthetic rubber and medicines and as a component of hypergolic fuels in space travel.
Reactions
At the amino group
Aniline is the simplest aromatic amine and only slightly soluble in water. An acid (for example hydrochloric acid) is added to aniline in order to promote or increase the solubility in water; this leads to the immediate formation of salt. The hydrochloride anilinium chloride is formed with hydrochloric acid :
If aniline is allowed to react with its salt (for example aniline hydrochloride ) in the heat, diphenylamine is formed :
The reaction of aniline with acetic anhydride results in N -phenylacetamide ( acetanilide ):
The representation of nitrobenzene from aniline is also possible. The aniline is converted to nitrobenzene via the intermediate stage nitrosobenzene with an oxidizing agent (such as hydrogen peroxide , mCPBA , potassium permanganate , chromium (VI) oxide or lead (IV) oxide ) .
The reaction of nitrosobenzene and aniline leads to azobenzene through elimination of water :
Direct nitration with nitrating acid leads to oxidation of the amino group. The desired nitroanilines are only obtained as acetanilide after the amino group has been protected beforehand.
If you reduce the nitroanilines now obtained with the help of a reducing agent (such as zinc in hydrochloric acid, sodium borohydride , lithium aluminum hydride or sodium sulfite ), you get the phenylenediamines :
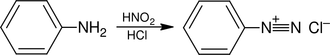
The reaction known as diazotization is also known. The aniline reacts with sodium nitrite in the presence of, for example, hydrochloric acid or with nitrous acid to form benzene diazonium chloride:
The benzenediazonium is a strong, unstable electrophile in the dye chemistry is a great application. The benzene diazonium chloride can react with water to form phenol with loss of nitrogen. A Sandmeyer reaction is also possible in the presence of copper (I) chloride and hydrochloric acid with the addition of heat - the chlorobenzene is also formed with loss of nitrogen. While retaining the two nitrogen atoms, the benzene diazonium ion can be reduced to phenylhydrazine , for example with sodium sulfite in an aqueous solution :
If the benzene diazonium chloride is allowed to react, for example, with an alkaline 2-naphthol solution ( ), the so-called Sudan I is obtained . This reaction is also known as the coupling reaction, more precisely as azo coupling :
Apart from the naphthols , other coupling reagents such as, for example, 1-naphthylamine can also be used. Because of the large number of possible coupling reagents, there are many important azo dyes , which is why they form the strongest class of dyes in terms of numbers.
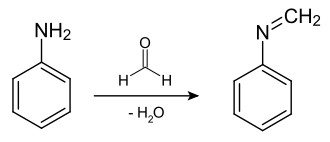
If aniline is allowed to react with formaldehyde (37%) according to the mechanism of imine formation, N -methylideneaniline is obtained :
First, the nitrogen of the amino group of the aniline attacks the aldehyde group ( carbonyl group ) of the formaldehyde . The double bonded oxygen of the aldehyde group becomes a single bond with a negative charge. The nitrogen is initially positively charged until it releases its proton to the negatively charged oxygen of the aldehyde group. Spontaneously or by adding a dehydrating agent, water is split off from the molecule ( hydroxyl group of the former formaldehyde + proton of nitrogen). A compound of the general structure R′ – N = CH – R ′ ′ is created. N -methylideneaniline belongs to the azomethine class of compounds (Schiff's bases) and, due to the methylidene group, has a very reactive point of attack for nucleophiles.
The reaction of aniline with benzaldehyde leads to benzalaniline (benzylidene aniline ):
On the ring
The sulfonation is an electrophilic aromatic substitution S E . Sulfur trioxide (SO 3 ) is introduced as an electrophilic particle , which is formed by the reaction of sulfuric acid with itself:
Formation of the electrophile ( sulfur trioxide ):
According to the principle of electrophilic substitution ( sulfonation ), sulfanilic acid can be synthesized.
Further electrophilic substitutions are also possible. For example halogenation , Friedel-Crafts alkylation and Friedel-Crafts acylation .
A catalytic hydrogenation of aniline in the presence of a catalyst leads to cyclohexylamine ( CHA ). (Noble) metals such as palladium are usually used as a catalyst for catalytic hydrogenation :
safety instructions
Aniline is a powerful blood poison. It oxidizes the red blood pigment hemoglobin to methemoglobin and thus prevents the transport of oxygen in the blood. The poison can be absorbed through swallowing, inhalation and through the skin. In the case of slight poisoning, the skin and fingernails turn blue ( cyanosis ), dizziness and agitation. Headaches, dizziness, impaired consciousness and shortness of breath occur at higher concentrations. The latter can cause death. Long-term symptoms of intoxication show up in weakness, loss of appetite and bladder cancer.
In 1981, aniline was also one of the causes of the mass poisoning that occurred in Spain through contaminated rapeseed oil ( Spanish oil syndrome ): rapeseed oil denatured with aniline for industrial purposes was redistilled and then sold as "olive oil" via street vendors. 20,000 people fell ill and more than 300 died. The exact causes of the poisoning have not yet been clarified.
See also
literature
- Karl Aloys Schenzinger : Aniline - novel of a dye . 1936.
- Christian Mähr : From alcohol to sugar - twelve substances that changed the world . Cologne 2010, ISBN 978-3-8321-9549-6 .
- Margarete Bruns: About azurite, indigo and aniline. The history of the blue color. In: Emil Ernst Ploß: A book of old colors. Technology of textile colors in the Middle Ages with an outlook on solid colors. 6th edition. Munich 1989, ISBN 978-3-89164-060-9 , pp. 14-20.
- A "contribution to the history of scientific discoveries" - the discovery of aniline by Friedlieb Ferdinand Runge . In: The Gazebo . Volume 4, 1863, pp. 64 ( full text [ Wikisource ]).
Web links
Individual evidence
- ↑ Entry on aniline. In: Römpp Online . Georg Thieme Verlag, accessed on May 19, 2017.
- ↑ a b c d e f g h i j Entry on aniline in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-26.
- ↑ Entry on aniline in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 62-53-3 or aniline ), accessed on September 14, 2019.
- ^ Hunnius Pharmaceutical Dictionary . 6th edition. Walter de Gruyter, Berlin / New York 1986, p. 68.
- ^ Henri A Favre, Warren H Powell: Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 . Ed .: The Royal Society of Chemistry. Cambridge 2014, ISBN 978-0-85404-182-4 , pp. 416, 668 .
- ↑ Otto Unverdorben: About the behavior of organic bodies in higher temperatures , Annalen der Physik und Chemie , 1826 , VIII , P. 397-410 ( limited preview in the Google book search).
- ↑ FF Runge: About some products of hard coal distillation , Annalen der Physik und Chemie , 1834 , XXXI , pp. 65-78 ( doi: 10.1002 / andp.18341070502 ; limited preview in the Google book search).
- ↑ J. Fritzsche: About the aniline, a new decomposition product of indigo , Annalen der Chemie , 1840 , 36 (1), pp. 84-90 ( doi: 10.1002 / jlac.18400360108 ).
- ↑ Dr. N. Zinin: Description of some new organic bases, represented by the action of hydrogen sulphide on compounds of hydrocarbons with subnitric acid , Journal for practical chemistry , 1842 , pp. 140–153 ( doi: 10.1002 / prac.18420270125 ; limited preview in Google Book search).
- ↑ AW Von Hofmann, JS Muspratt: Neue Bildungsweise des Anilins , Annalen der Chemie , 1845 , 53 (2), pp. 221–229 ( doi: 10.1002 / jlac.18450530206 ; limited preview in the Google book search).
- ^ AW von Hofmann: About a safe reaction to benzene , Liebigs Annalen , 1845 , 55 (2), pp. 200–205 ( doi: 10.1002 / jlac.18450550205 ).
- ↑ In: Ber. dt. Chem. Ges. , 23, 1890, p. 3043, gallica.bnf.fr
- ↑ H. Wittcoff et al .: Industrial Organic Chemicals . 2004, Chapter 7, p. 294.
- ↑ E. Gelpi et al .: The Spanish Toxic Oil Syndrome 20 Years after Its Onset: A Multidisciplinary Review of Scientific Knowledge . In: Environmental Health Perspectives , 2002, 110 (5), pp. 457-464, PMID 12003748 ; PMC 1240833 (free full text).