fungicide
A fungicide is a chemical or biological agent that kills fungi or their spores, or prevents them from growing while it is effective. The property is called fungicidal ("fungicidal"), the process fungicidal .
Areas of application
Fungicides are mainly engaged in agriculture as pesticides applied. They are also used to combat harmful fungi (e.g. mold ) on wood , paint, textiles , on walls ( dry rot ) and on food . The term antimycotics is more common for fungicides that are used in medicine (e.g. against skin fungi ) . In contrast to agriculture, fungicidal biocides are also used as disinfectants . In this area, a clear distinction is made between fungicidal and sporicidal effects, since rendering (mold) spores harmless requires significantly more aggressive chemicals and longer exposure times than killing the biologically active mycelial cells and spore-forming cells.
history
In the past, crop failures due to fungal growth were not uncommon.
In the years 1845/46 there was a total failure of the potato harvest in Ireland due to fungal attack. About a million Irish died in the famine, and two million Irish emigrated to the United States.
The same fungus led to severe crop failures in the potato harvest in Germany in 1916/1917 (" turnip winter ").
There were also major plagues from fungal infestation in viticulture . In France, 80% of the harvest was destroyed in 1848.
In the early days , sulfur , ash , lime or urine were used to protect the plants from infestation. Glauber's salt was used in 1635 and copper sulphate containing arsenic in 1740 (prohibited in 1786 due to the risk of poisoning).
In the 19th century, plants were protected against powdery mildew with metal salts such as Bordeaux broth made from copper sulfate and calcium oxide and lime sulfur broth . Mercury and organic mercury compounds were also used between 1897 and 1930 .
Since 1930 dithiocarbamates have been used in the USA , from 1946 dinocarb (first fungicide with selective action against fungal growth) against molds.
Between 1960 and 1980, many new classes of compounds for fungicides followed, such as carboxanilides (e.g. flutolanil ), piperazines ( triforin ), phthalimides ( captan , captafol ), morpholines ( tridemorph ), imidazoles ( prochloraz ), benzimidazoles ( carbendazim , benomyl ) and triazoles ( Propiconazole ). Since 1990 the azoles ( bitertanol , hexaconazole ) and later the strobilurins ( azoxystrobin ) came onto the market. These latter groups very quickly entered the fungicide market.
Mode of action
Fungi do not have chlorophyll and therefore cannot synthesize carbohydrates from carbon dioxide and water. They live on the tissue of other organisms and get their food from the host's body. Fungal enzymes form fungal filaments that can dissolve the host's cell walls. Fungal spores are scattered in the air and in the soil. If they encounter a favorable environment, new fungal threads will grow quickly.
Fungicides can have a protective , curative or eradicative effect. Protective fungicides prevent spore germination or the penetration of the fungus into the plant tissue. This can be done by acting directly on the spore ( sporicidal effect) or by changing the physiological conditions on the leaf. When using protective fungicides, several sprayings are often necessary to prevent infection during the risk period . Overall, this leads to high application rates and high labor costs.
Curative and eradicative fungicides have also been available since the mid-1980s. Curative fungicides can stop infection in the early stages. Eradicative fungicides can successfully combat fungal infestation even when symptoms of the infestation are already visible. So far, there are eradicative active ingredients only for combating ectoparasitic fungi such. B. powdery mildew .
Place of application
A distinction is made between leaf fungicides , soil fungicides and seed dressings according to the place of application or the method of application .
Foliar fungicides are sprayed or dusted on the above-ground parts of the plant, soil fungicides are introduced into the soil .
The pickling as a seed treatment to fungal spores on the seed kill and prevent infection of the seedling. The seeds can be dipped in fungicide solution or sprayed with it (wet dressing) or brought into contact with fungicide powder (dry dressing ) . Nowadays, practically all grain seeds are dressed before sowing. Until 1982, pickling agents containing mercury were permitted in Germany .
Pick-up and transport
Systemic xylem distribution in the plant
Fungicides that are absorbed through the leaf or roots and displaced by the plant's transport system are called systemic fungicides. Young shoots in particular are well protected by systemic fungicides.
Active ingredient groups such as triazoles , benzimidazoles and morpholines (e.g. Corbel or Tridemorph ) are fully water-soluble and do not create a depot. They are systemically distributed "upwards" (= acropetal) with the water flow in the xylem.
An already existing strong disease infestation inhibits the distribution in the plant. The upward movement of the active ingredients creates a thinning effect in the older parts of the plant. The general statement that systemic active ingredients are distributed “everywhere in the plant” is wrong, especially in cereals. Due to the habit of the monocot plants, they are only distributed from bottom to top. For practical use, this means that the plant must be well wetted with fungicide, especially in the lower area. Special nozzles are used for this.
Subsystemic distribution
Local systemic active ingredients are absorbed into the plant, but are only distributed there to a small extent. Especially the active substance group of the imidazoles (e.g. prochloraz ) penetrates the tissue, remains there and is not transported in the water flow. So there is no dilution.
Depot effect and translaminar moisture penetration
Various groups of active ingredients, in particular the strobilurins , but also the anilino-pyrimidines, quinolines and oxazolidine- diones create a depot in the wax layer from which the active ingredients slowly and continuously "moisturize" the leaf tissue translaminar, which has a long lasting effect.
Contact effect
The non-systemic fungicides or contact fungicides do not penetrate the plant, but remain on its surface. If the plant forms new leaves or if the fungicide was washed off by rain, it must be sprayed again.
Fungicide resistance
Contact fungicides are becoming increasingly important in the context of the resistance problems of fungicides in cereal cultivation, and they also play a very important role in potato cultivation.
Active ingredients
Fungicides can be inorganic , organometallic or organic chemicals or organisms .
Inorganic fungicides are, for example, Bordeaux broth (Cu (OH) 2 · CaSO 4 ) or copper oxychloride (basic copper chloride) (3 Cu (OH) 2 ) · CuCl 2 · n H 2 O). Copper (II) ions are released from these fungicides , which act as enzyme poisons in the fungal spores and can thus prevent germination. Colloidal , pure sulfur (network sulfur ) is also an inorganic fungicide. It oxidizes to sulfur dioxide on the plant surface , which inhibits spore germination. Inorganic fungicides still make up about half of the fungicides sold. They can also be used in organic farming , even in biodynamic farming (Demeter).
Organometallic fungicidal active ingredients such as the very toxic and environmentally harmful mercury and organotin are banned today.
The group of organic fungicidal active ingredients is very heterogeneous and difficult to understand. In the case of cereal fungicides, which are important in terms of quantity, active ingredients from the classes of azoles , morpholines and strobilurins are used today.
In Germany, a biological agent containing spores of the parasitic fungus Coniothyrium minitans is currently approved for combating Sclerotinia fungi (e.g. white stalk in rapeseed ).
Active ingredient groups (overview)
The table is arranged according to the shares in global sales (see right column).
group Fungicide group Contains Example connection % of world sales
(S. Group, 2004)DMI fungicides Triazoles 
Epoxiconazole27.7 QoI fungicides Strobilurins 
Trifloxystrobin19.1 Dithiocarbamates Dithiocarbamates 
Maneb7.2 Copper & sulfur Network sulfur 
4.8 MBC fungicides Benzimidazoles and thiophanates 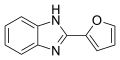
Fuberidazole3.6 Chloronitrile Chlorothalonil 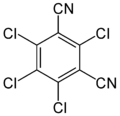
Chlorothalonil3.3 Dicarboximide Dicarboximide 
Chlozolinate2.9 Phenylamides Acetylalanine 
Benalaxyl2.8 Amines Morpholines, piperidines 
Fenpropimorph2.7 AP fungicides Anilinopyrimidines 
Cyprodinil2.6 MBI fungicides 
Tricyclazole2.6 SDHI Carboxamides 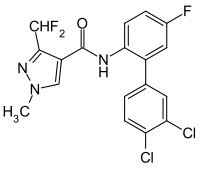
Bixafen1.8 Decoupler 
Fluazinam1.8
Active ingredient groups in grain production
Contact agents
Belong to this group
- Wetting sulfur : elemental sulfur against various types of mildew (cereals, vegetables, wine, fruit). Penetrates the fungal cells and disrupts the respiratory chain in the mitochondria.
- Guazatin : In seed dressings against Septoria nodorum and snow mold
- Chlorthalonil : against Septoria, Helminthosporium leaf drought (HTR), Ramularia. Increasing importance against resistant Septoria strains.
- Iprodione : against Septoria and HTR, also against canola cancer in canola
- Anilazine : against Septoria and HTR, currently not approved, previously liquid in Dyrene.
Azoles
The largest and (besides strobilurins) the most important group of fungicides. It is divided into the subgroups of benzimidazoles , triazoles and imidazoles, depending on the properties of action .
Benzimidazoles ("MBC")
Mode of action in fungal metabolism: Inhibition of cell division (mitosis) by destroying the building blocks of the spindle apparatus. Only one point of attack in the metabolism of the fungus, therefore high risk of resistance. This group includes a. Active ingredients particularly effective against strawberries and snow mold. They have a healing (curative) but no preventive (protective) effect. Active ingredients are u. a.
- Carbendazim (e.g. Harvesan fungicide and seed dressings)
- Thiophanate methyl (e.g. cercobin)
- Fuberidazole (e.g. in seed dressings)
- Benomyl (no longer approved)
Carbendazim is the actual active ingredient. Thiophanate methyl and benomyl are converted to carbendazim in the plant.
Triazoles
All triazoles are systemically distributed in the plant and have different preventive (= protective), healing (= curative) and stopping (= eradicative) effects. They belong to a subgroup of SBI fungicides (Sterol Biosynthesis Inhibitors). This subgroup is referred to as DMI fungicides (demethylase inhibitors). It inhibits the enzyme C14 demethylase, which demethylates lanosterol in the ergosterol biosynthetic pathway of fungi. Ergosterol fulfills the same function in fungal plasma membranes as cholesterol in animal and phytosterols in vegetable plasma membranes.
Growth regulatory side effects: Triazoles and especially the strobilurins (see there) all have a more or less strong positive influence on the chlorophyll content, longer assimilation time, improved photosynthesis and growth in length (shortening). They inhibit the formation of the maturation hormones. This leads to a slower ripening process and thus to longer grain storage but also to a later harvest date and is known as the “green effect”.
The triazoles include u. a. the following active ingredients: Propiconazole (e.g. Desmel), Flusilazole (e.g. Capitan), Metconazole (Caramba), Epoxiconazole (e.g. Opus), Fluquinconazole (e.g. Flamenco), Tebuconazole (e.g. Folicur, many stains), prothioconazole (e.g. Proline), difenoconazole (e.g. Spyrale), cyproconazole (e.g. Radius), triadimenol (e.g. Bayfidan), bromuconazole (e.g. granite) , Myclobutanil . Fenbuconazole and bitertanol are no longer permitted (e.g. earlier in Baycor).
Imidazoles
Subsystemic distribution. Two important active ingredients are prochloraz (e.g. Mirage) and imazalil (used in pickling against streak disease in barley).
Morpholines
Morpholines are another subgroup of SBI fungicides (sterol biosynthesis inhibitors). They inhibit two enzymes involved in the structure of the cell wall (a reductase and an isomerase). The resulting holes in the cell wall lead to rapid drying out, especially in the case of powdery mildew fungi (= strong eradicative effect). Due to the two points of attack, the risk of resistance is very low or even absent. The group includes the active substances fenpropimorph and fenpropidin .
Strobilurins
They form an active ingredient depot in the wax layer with subsequent translaminar distribution and intervene in the mitochondria in the energy metabolism (respiratory chain) of the mushrooms. They only block one enzyme there and are therefore at high risk of resistance (monogenetic resistance to powdery mildew and Septoria). The strobilurins include u. a. the active ingredients picoxystrobin , azoxystrobin , kresoxim-methyl , pyraclostrobin , fluoxastrobin , trifloxistrobin , dimoxystrobin .
Quinolines
Quinolines, like strobilurins, form a depot in the wax layer and show a translaminar distribution. They have no healing (curative) effect, but a relatively long-lasting effect. Active ingredients: Quinoxyfen (e.g. Fortress) and Proquinazid .
Anilino pyrimidines
The anilino-pyrimidines, like strobilurins, also form a depot in the wax layer with subsequent translaminar and systemic distribution. They block the synthesis of the amino acid methionine in the fungal metabolism and thus inhibit penetration into the leaf (formation of appressorias, penetration) and growth in the leaf tissue (formation of haustoria). Active ingredients: cyprodinil , mepanipyrim and pyrimethanil
Oxazolidine dione
Like strobilurins, this group of active substances also forms a depot in the wax layer with subsequent translaminar distribution. It prevents the spores from germinating by inhibiting the respiratory chain in the mitochondria. Active ingredient: famoxadone .
Carboxamides
In 2003, BASF launched the active ingredient boscalid on the market. In 2011 and 2012, further fungicides with active ingredients from the group of carboxamides (carboxamides) were approved. For Bayer Crop Science these were Aviator Xpro, Input Xpro and Skyway Xpro (all with the active ingredient Bixafen ) and for BASF the Adexar (with the active ingredient Fluxapyroxad ). Syngenta is also working on a new fungicide isopyrazam from this group of active ingredients. It was first registered in England in 2010.
Carboxamides inhibit complex II of the respiratory chain , succinate dehydrogenase . They are often used in combination with azoles in order to avoid the development of resistance as long as possible. Since carboxamides mainly suppress the germination of fungal spores, the curative azoles are a suitable supplement.
Consumption and production quantities
In Germany, around 10,000 tons of fungicides ( active ingredients ) are sold and used in crop protection every year . In 2017, 13,271 tons of fungicidal active ingredients or 33,376 tons of fungicides (including bactericides and virucides ) were released. This corresponds to around a quarter of the total amount of plant protection products . In Austria, the annual fungicide consumption is around 1400 tons.
In Central Europe, the proportion of fungicides in the crop protection products sold is relatively large. Because of the humid climate and the densely sown grain stocks, the risk of fungal infections is greater here than in other parts of the world.
| fungicide | Quantity produced in tons | Sales in million euros |
|---|---|---|
| Inorganic products | 13,989 | 22.2 |
| Dithiocarbamates | 7,313 | 52.9 |
| Benzimidazoles | 295 | 1.8 |
| Diazoles, triazoles | 7,428 | 275.6 |
| Morpholines | 1,493 | 70.9 |
| Other fungicides | 13,750 | 417 |
Recent developments
There are mushrooms that produce toxins against other mushrooms. W. Steglich and T. Anke were able to isolate two important active substances in 1977. These active ingredients are called strobilurins .
The BASF and Zenca ( Syngenta ) examined these ingredients with respect to their use in crop protection. The natural strobilurins turned out to be very unstable. The active ingredients quickly lose their activity through air and light. In 1996 the active ingredient azoxystrobin with good resistance and good fungicidal effect was found and brought onto the market. This fungicide is used on Septoria and Rost and is used in the cultivation of grain, fruit and vegetables.
In the same year, BASF found the active ingredient kresoxim-methyl against powdery mildew on grain, pome fruit and grapevines. In 2003, BASF introduced another new active ingredient, dimoxystrobin, onto the market.
These active ingredients resulted in an increased yield of around 10% and made the plants stay green longer. The biochemical chains of effects in the inhibition of the spread of fungi can now be better analyzed. The biochemical substance ubiquinone or coenzyme Q , which is responsible for electron transport, is hindered by strobilurins. This means that mushrooms cannot gain energy.
In a few years - up to the year 2000 - the total turnover of strobilurins could be increased to 14.8% of the world production of fungicides.
Other new active ingredients have also been found in recent years. The names of these substances are, for example, famoxadone and cyazofamide . Bayer has developed the active ingredients prothioconazole and spiroxamine to combat steroid biosynthesis in mushrooms .
A difficulty in developing new fungicides is likely to be the correct advice to farmers, the precise preparation of toxicological studies, the correct determination of the special area of application, the highlighting of the improved useful spectrum in comparison to other or older agents. According to the 2007 production statistics for Germany, the fungicide groups of dithiocarbamates, triazoles, morpholines, benzimidazoles and even the inorganic fungicides also have considerable market potential. Convincing for the consumer would be the lowest possible use of fungicides per hectare with very good activity and no side effects.
Whereas in the past 2–5 kg of sulfur had to be used as a fungicide for one hectare in order to keep the field free of fungi, dithiocarbamates only needed 1500–2000 g per hectare. With the Triadimefon , consumption could be reduced to only 50–120 g / hectare.
Admission
Before they are launched on the market, fungicides that are to be used as pesticides undergo an official approval process in which, among other things, their effectiveness against harmful pathogens and environmental compatibility must be proven.
The approved fungicides can be found online. The preparations and active ingredients approved in Switzerland can be viewed at the Federal Office for Agriculture , in Austria the Agency for Health and Food Safety (AGES) is responsible and in Germany the Federal Office for Consumer Protection and Food Safety. The Directorate-General for Health and Food Safety provides information on the active ingredients approved in the EU .
In 2019, due to new studies on chlorothalonil and the metabolites in groundwater (and subsequently in drinking water), the substance's approval was withdrawn in the EU and Switzerland and its sale was banned from autumn 2019. Remaining stocks may still be used up until the end of May 2020.
See also
literature
- Michael Henningsen: Modern Fungicides: Fighting Fungi in Agriculture. In: Chemistry in Our Time . Vol. 37, No. 2, 2003, pp. 98-111, doi: 10.1002 / ciuz.200300283 .
Web links
Individual evidence
- ↑ a b c d e f Michael Henningsen: Modern Fungicides: Fighting Fungi in Agriculture . In: Chemistry in Our Time . tape 37 , no. 2 , 2003, p. 98–111 , doi : 10.1002 / ciuz.200300283 .
- ↑ a b Ullmann's Encyclopedia of Technical Chemistry, 4th Edition, Volume 18, Keyword: Plant Protection Products, Toxicology, p. 4 ff.
- ^ Roland Dittmeyer, Wilhelm Keim, Gerhard Kreysa, Karl Winnacker, Leopold Küchler: Chemische Technik. Volume 8, Nutrition, Health, Consumer Goods. 5th edition. Wiley-VCH, 2004 ISBN 3-527-30773-7 , pp. 218-223.
- ^ Günter Vollmer and Manfred Franz: Chemical products in everyday life , Georg Thieme Verlag Stuttgart, 1985, pp. 415-416, ISBN 3-13-670201-8 .
- ↑ Wolfgang Krämer, Ulrich Schirmer, Peter Jeschke, Matthias Witschel: Modern Crop Protection Compounds: Fungicides . tape 2 . Wiley-VCH, 2011, ISBN 978-3-527-32965-6 , pp. 1231 ( limited preview in Google Book search).
- ↑ Anna-Katharina Girbig: Investigation of selected pesticides in the catchment area of the Fuhse: inventory 2011 . Ed .: NLWKN . Hildesheim 2013, DNB 1038716586 , p. 8th f . ( PDF ).
- ↑ BASF: Boscalid - a modern fungicide for special crops ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. , accessed June 4, 2012.
- ↑ Adexar data sheet
- ↑ Chemie.de: Syngenta receives first approval for isopyrazam plant protection product in Europe , April 1, 2010.
- ^ Stephan Weigand: Fungicide resistance in cereals - Current situation in Bavaria. March 2011 ( yumpu.com ).
- ↑ Federal Office for Consumer Protection and Food Safety : Sales of plant protection products in the Federal Republic of Germany . September 18, 2018, accessed September 23, 2018.
- ↑ Federal Statistical Office, Series 4, Series 3.1, year 2008.
- ^ Directorate-General for Health and Food Safety of the European Commission: EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on February 17, 2016.


