Salts
As salts refers to chemical compounds or crystalline substances, the positively charged ions ( cations ) and negative ions ( anions ) are constructed. Ionic bonds exist between these ions . In the case of inorganic salts , the cations are often formed by metals and the anions are often formed by non-metals or their oxides. As a solid, they together form an ion lattice . As organic salts refers to all compounds in which at least one anion or cation an organic compound is; with the exception of the carbonates , which are derived from carbonic acid (H 2 CO 3 ) , which is by definition inorganic .
Inorganic salts

In the narrowest sense, salt means sodium chloride (NaCl, table salt ). In a broad sense, all compounds that are built up from anions and cations like NaCl - such as calcium chloride (CaCl 2 ) - are called salts. Sodium chloride is made up of the cations Na + and anions Cl - . The salt calcium chloride is formed from Ca 2+ and Cl - . The formulas NaCl and CaCl 2 are the ratio formulas of the compounds (Na: Cl = 1: 1, or Ca: Cl = 1: 2). Ions can be mono- or multivalent , that is, they can carry one or more positive or negative charges.
The ratio formula of a salt is determined by the number of charges of the ions, since positive and negative charges have to compensate each other. Ratio formulas of salts are in clear contrast to formulas of compounds such as water (H 2 O) or methane (CH 4 ), which are molecular compounds.
In the case of inorganic salts, ionic bonds exist between the ions. A very large number of ions form an ion lattice with a specific crystal structure while observing the respective ratio formula . The figure on the right shows a small section of the structure of a sodium chloride crystal lattice. Since there are many different cations and anions, a large number of different salts are known. Some of the ions are listed in the tables below.
Ions in salts can also consist of more than one atom. They are called complex ions. Examples of complex anions are the nitrate anion (NO 3 - ) and the sulfate anion (SO 4 2− ). In complexes, an atom is the central atom to which other atoms (or groups of atoms) are attached and are called ligands . In the two examples, N and S are the respective central atom, ligands are oxygen atoms in both cases (oxo complexes). The central atoms and their ligands are linked to one another by covalent bonds . Ionic bonds exist only between the anions and cations. Among the nitrates, for example, the salt sodium nitrate (NaNO 3 ) is known, among the sulfates sodium sulfate (Na 2 SO 4 ).
Cations are mostly formed by metals and their salts are called metal salts . The complex cation ammonium (NH 4 + ) consists of non-metals with nitrogen as the central atom and hydrogen as ligands. Ammonium ions, for example, form the salt ammonium sulfate ((NH 4 ) 2 SO 4 ). There are organic compounds analogous to ammonium compounds ( quaternary ammonium compounds ), which are described in more detail below.
In the case of polyvalent oxo complexes, OH groups can also occur as ligands, e.g. B. the salt sodium hydrogen sulfate (NaHSO 4 ). Analogous salts are also known under the phosphates: In addition to sodium phosphate, there are also the salts disodium hydrogen phosphate and sodium dihydrogen phosphate . The OH groups as ligands cannot be recognized directly from the usual formula notation (formula unit ) for these compounds. The formula units of such salts are derived from the traditional notation for acids such as sulfuric acid (H 2 SO 4 ) and phosphoric acid (H 3 PO 4 ).
Transition metals can not only form cations, but also anions as oxo complexes. Chromium can form the chromates ([CrO 4 ] 2− ), the anion in potassium chromate (K 2 [CrO 4 ]) and manganese the permanganates ([MnO 4 ] - ), the anion in potassium permanganate (K [MnO 4 ]) .
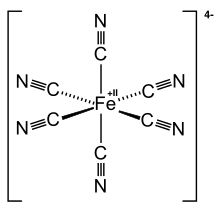
Complex anions can also have metals as a central atom. In the case of potassium hexacyanoferrate (II) (K 4 [Fe (CN) 6 ]), the iron ion Fe 2+ together with six cyanide groups (CN - ) together form a stable anion with four negative charges. In the salt there are ionic bonds between potassium ions and the hexacyanoferrate (II) anion. Similarly, the iron ion Fe 3+ potassium hexacyanoferrate (III) (K 3 [Fe (CN) 6 ]) also forms a complex salt. In the case of K 3 [Fe (CN) 6 ], the iron ion Fe 3+ together with six cyanide groups (CN - ) together form a stable anion with three negative charges.
Examples of cations and anions
|
|
|||||||||||||||||||||||||||||||||||||||
Properties of salts
- Many salts are solids at room temperature with relatively high melting points . Many salts are quite hard and brittle and have smooth breaking edges when mechanically processed. These properties are quite typical for solids, which are built up by an ion lattice and therefore form crystals . But not every crystalline substance is a salt. Thus forming sugar ( sucrose ) and crystals, but has no ionic lattice and not one of the salts.
- Many salts are soluble in water and insoluble in most organic solvents . In the case of water-soluble salts, the water overcomes the lattice energy of the ion lattice through hydration . If the hydration energy is similar to or greater than the lattice energy, the salt is moderately or readily soluble. In solutions, the individual ions of water molecules are very tightly and intensively coated. As a reaction, this is often represented in chemistry as follows: The (s) indicates a solid and (aq) indicates that the ion is hydrated .
- Dry salt crystals are electrical insulators . Salt melts and aqueous solutions, on the other hand, conduct the electric current due to their freely moving ions as charge carriers ; they are electrolytes .
- Dissolving salts in water can change the pH of the respective solution. If the salt does not affect the value, one speaks of neutral salts . Sodium chloride is also one of the neutral salts. Other salts raise or lower the pH. One speaks of basic or acidic salts . How a certain salt reacts is difficult to estimate from the composition of the compound. In principle, however, the following applies: Anions (acid residues) of strong acids usually react neutrally. Acid residues from weak acids usually have a basic reaction. The behavior of phosphates is exemplary of salts, of which polyprotonic acids are known . The dissolution of salts in aqueous solutions of organic molecules, such as. B. of biomolecules, can lead to denaturation of the biomolecules or cause the precipitation of the macromolecules. This effect of salts is characterized by the so-called Hofmeister series .
Other cations and anions
- Metal oxides make up a large part of the earth's crust and can also be viewed as salts. The anion O 2− ( oxide ion ) occurs as such only in the solid or molten state; it is not known in aqueous solutions. The oxygen in the oxide ion has an oxidation number of −2. The oxidation number of the metals thus determines the ratio formula of the respective compound: M I 2 O, M II O, M III 2 O 3 . If an oxide is water-soluble, a specific chemical reaction takes place, for example: Sodium oxide reacts with water to form hydroxide ions to form caustic soda . Calcium oxide (CaO), also known as quick lime , reacts in a similar way to form slaked lime (Ca (OH) 2 ). Many oxides do not react with water. The iron (III) oxide (Fe 2 O 3 ) is not a water-soluble compound.
- Sulphides: Minerals are often found in nature as sulphides (S 2− ), e.g. B. pyrite and copper luster . Sulphides can also be viewed as salts. Sodium sulfide (Na 2 S) is a soluble salt; most sulfides, such as zinc sulfide (ZnS) and copper (II) sulfide (CuS), are practically insoluble in water. In analytical chemistry , the different (poor) solubility of various metal sulfides is used to separate the elements (in the separation process of the hydrogen sulfide group ).
Crystal water
In addition to the ions, many salts also contain water molecules in certain quantities , the so-called crystal water . It is also specified in the ratio formula , as here in the example of sodium sulfate deca hydrate : Na 2 SO 4 · 10 H 2 O.
Double salts
In addition to salts with only one type of cation (M), salts with two different cations are also known. These salts are called double salts , like alums with the general composition M I M III (SO 4 ) 2 . Example: aluminum potassium sulfate dodecahydrate (KAl (SO 4 ) 2 · 12 H 2 O).
Limits of the term salts
- Substances are only salts if there are ionic bonds between the particles of the compound. However, it is not easy to deduce whether this type of bond exists. While calcium oxide (CaO) has ionic bonds, chromium (VI) oxide (CrO 3 ) only has covalent bonds between chromium and oxygen atoms; so it is not salt. Therefore, in these cases it is better to speak of metal oxides instead of salts .
- For historical reasons, salts are usually understood as chemical compounds , as they have a defined composition of different chemical elements. However, mixed crystals are known from two salts that are not stoichiometrically composed: Potassium permanganate (K [MnO 4 ]) forms mixed crystals with barium sulfate (Ba [SO 4 ]) in almost any proportions (even if only up to a certain maximum of barium sulfate ), as the components have similar crystal structures and lattice spacing. A chemical similarity of the compounds involved or an equal valency is not necessary for the formation of mixed crystals.
Organic salts
In addition to the inorganic salts described above, there are also numerous salts of organic compounds . The anions of these salts are derived from the organic acids . The salts of the carboxylic acids , such as acetic acid , of which many salts, the so-called acetates (CH 3 COO - ) are known, are important here. The salt sodium acetate can be formed with Na + or the copper acetate with Cu 2+ . Acetic acid is a monocarboxylic acid (has only one -COOH group) and only forms monovalent anions. Citric acid is a tricarboxylic acid (has three -COOH groups) and can form trivalent anions; their salts are called citrates . For example, the salts sodium citrate and calcium citrate are known . Many acetates and citrates form crystals, but that is not the real reason for calling them salts. The real and only reason is due to the presence of ionic bonds between anions and cations. Covalent bonds exist within the ions of organic compounds .
The salts of carboxylic acids , which are among the fatty acids, are of practical importance . The sodium or potassium salts of fatty acids are called soaps . Mixtures of different fatty acid salts are present in soaps. They find practical use as curd soap or soft soap . As a concrete example, palmitic acid forms salts, which are called palmitates . Salts based on such large organic molecules are usually not crystalline.
Analogous to the inorganic sulfates (SO 4 2− ) there are also organic sulfates (RO-SO 3 - ), such as sodium lauryl sulfate , which are used as surfactants in shampoos and shower gels. Salts, the alcoholates , of alcohols are also known. Alcohols are extremely weak acids and are therefore almost never called that. Compounds of the form RO - M + (M = metal) can be obtained under aggressive reaction conditions . In analogy to many inorganic oxides (MO), alcoholates react with hydrolysis on contact with water and the corresponding alcohols are formed.
| Hydrolysis of oxidic salts | |
|---|---|
| Sodium ethanolate | |
| Sodium oxide | |
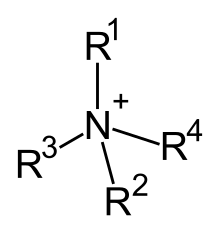
Among the organic cations, the compounds analogous to the ammonium cation (NH 4 + ) are important. They are commonly called quaternary ammonium compounds . In these compounds, the nitrogen atom usually carries four alkyl groups (R-) and one positive charge. For example, the alkylammonium compound cetyltrimethylammonium bromide is an organic ammonium compound in which a bromine atom is present as an anion. Ammonium compounds with three short and one long alkyl groups are of practical importance, since these cations show the properties of surfactants in aqueous solution . Compounds of this kind also play an important role in the metabolism of living things, such as choline .
In principle, every organic amine can become a cation by taking up a proton (H + ). Analogously to the reaction of ammonia (NH 3 ) to form the ammonium ion (NH 4 + ), for example, a primary amine (R-NH 2 ; R = organic radical) reacts to form the cation R-NH 3 + . Since such compounds are usually more polar and therefore more easily soluble in water than the original substances, nitrogen-containing medicinal products (active pharmaceutical ingredients) , for example, are converted into salts, the so-called hydrochlorides , by adding hydrochloric acid . This will facilitate their absorption into the body. In contrast to amines, hydrochlorides can be more easily purified by recrystallization . Analogously, amines form hydrobromides with hydrogen bromide and hydrofluorides with hydrogen fluoride .
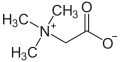
In addition to molecules that have a positive or negative charge, there are also molecules that have a negative and positive charge. They are called inner salts or zwitterions . The betaine group is one of the inner salts, the simplest compound of which is betaine .
The amino acids have a carboxy group (-COOH) and an amino group (-NH 2 ) and can thus react acidic and basic. In an internal neutralization , an anionic (-COO - ) and a cationic (-NH 3 + ) group and thus a zwitterion are formed. The simplest amino acid is glycine , which is readily soluble in water . In contrast to other ions dissolved in water, zwitterions show poor (no) electrical conductivity. ( Ampholyte )
Examples of organic cations and anions
| Anions of organic compounds | ||
|---|---|---|
| Substance group | example | structure |
| Carboxylic acid salts | Acetates | |
| Palmitates | ||
| Citrates | ||
| organic sulfates | Lauryl sulfate | |
| Alcoholates | Ethanolates | |
| Cations of organic compounds | ||
| Substance group | example | structure |
|
quaternary ammonium compounds |
Cetyltrimethylammonium | |
| Choline | ||
|
organic ammonium compounds |
Salts of aniline , e.g. B. aniline hydrochloride |
|
| Internal salts: cation and anion in one molecule | ||
| Substance group | example | structure |
| Betaines | Betaine | |
| amino acids | Alanine | |
Manufacture of inorganic salts
Reactions of acids and bases
Salts are formed when acids react with bases (Greek base ; Arrhenius: bases are the basis for salts). The oxonium ion of the acid and the hydroxide ion of the base form water ( neutralization ). Some salts are sparingly soluble in water and directly form the solid. The salt is usually in solution and can be obtained as a solid by evaporating the water.
| Acid + base → salt + water |
|---|
|
Hydrochloric acid + sodium hydroxide solution → sodium chloride + water |
|
Sulfuric acid + barium hydroxide → barium sulfate + water |
From other salts
Some salts can be obtained from two other salts. If you mix aqueous solutions of two salts, a third salt can form as a solid. This is only possible if the third salt is less soluble than the other two.
| Saline A + Saline B → Salt C + Saline D |
|---|
|
Sodium chloride + silver nitrate → silver chloride + sodium nitrate |
|
Calcium chloride + sodium carbonate → calcium carbonate + sodium chloride |
Reaction of oxides
As described above, many metal oxides tend to form hydroxides with water. Metal oxides that are "insoluble" (= stable) in pure water also react under acidic conditions. Many salts, such as copper sulfate, can be obtained in this way.
| Metal oxide + acid → salt + water |
|---|
|
Copper (II) oxide + sulfuric acid → copper sulfate + water |
Other reactions
The ions in the reactions described above are not formed first, but exist before a new salt is formed. If no or not all ions with the necessary charge are present in reactions to form a new salt, redox reactions take place. In this way, salts can be obtained from elemental metals and non-metals . Reactions of this type are described in more detail under the salt formation reaction .
See also
Web links
Individual evidence
- ↑ Entry on salt . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.S05447 Version: 2.2 ..
- ↑ Hans-Dieter Jakubke, Ruth Karcher (Ed.): Lexikon der Chemie. Spectrum Academic Publishing House, Heidelberg 2001.