acetone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | acetone | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 3 H 6 O | ||||||||||||||||||
| Brief description |
colorless liquid with a sweet odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 58.08 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
0.79 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
−95 ° C |
||||||||||||||||||
| boiling point |
56 ° C |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| solubility |
miscible with water and many organic solvents |
||||||||||||||||||
| Dipole moment | |||||||||||||||||||
| Refractive index |
1.3588 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
DFG / Switzerland: 500 ml m −3 or 1200 mg m −3 |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Acetone or acetone [ at͡səˈtoːn ] is the common name for the organic-chemical compound propanone or dimethyl ketone . Acetone is a colorless liquid and is used as a polar, aprotic solvent and as a starting material for many syntheses in organic chemistry. With its structural feature of the carbonyl group (> C = O), which carries two methyl groups , it is the simplest ketone .
Extraction and presentation
Acetone was first produced in 1606 by Andreas Libavius by heating lead (II) acetate . In 1661 Robert Boyle was able to win it through the dry distillation of wood. It was first described in 1610 in the Tyrocinium Chymicum by Jean Beguin . Until the middle of the 20th century, the acetone-butanol-ethanol fermentation discovered and patented by Chaim Weizmann was an important process for the production of acetone as well. The anaerobic bacterium Clostridium acetobutylicum was used for industrial production .
The most important manufacturing process of acetone today is the cumene hydroperoxide process , which is also known as the Hock phenol synthesis :
Here benzene and propene are first converted into isopropylbenzene ( cumene ) by a Friedel-Crafts alkylation in an acid . This then reacts with oxygen in a radical reaction to form the hydroperoxide , which decomposes to phenol and acetone during acidic processing .
The dehydrogenation or oxydehydrogenation of isopropanol serves as a further manufacturing process .
Another way of producing acetone is to heat calcium acetate ( 1 ), where it breaks down into acetone ( 2 ) and calcium oxide ("lime salt distillation").
This process goes back to the above-mentioned historical synthesis by Libavius in 1606.
properties
Acetone is a colorless, low-viscosity liquid with a characteristic, slightly sweet odor, highly flammable and forms an explosive mixture with air. The boiling point at normal pressure is 56 ° C. It is miscible with water and most organic solvents in all proportions. The acetone molecule shows keto-enol tautomerism ; its pK s value is 20. acetone may be due to its polar carbonyl group with cations also form complex compounds.
The compound forms azeotropically boiling mixtures with a number of other solvents . The azeotropic compositions and boiling points can be found in the following table. No azeotropes are formed with water , ethanol , 1-propanol , 2-propanol , n-butanol , benzene , toluene , ethylbenzene , diethyl ether , ethyl acetate and acetonitrile .
| Azeotropes with various solvents | ||||||||||||
| solvent | n -pentane | n -hexane | n -heptane | Cyclohexane | Methanol | chloroform | Carbon tetrachloride | Diisopropyl ether | Methyl acetate | |||
| Content of acetone | in% | 21st | 59 | 90 | 67 | 88 | 22nd | 89 | 61 | 50 | ||
| boiling point | in ° C | 32 | 50 | 56 | 53 | 55 | 64 | 56 | 54 | 55 | ||
Thermodynamic properties
According to Antoine, the vapor pressure function results from log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 4.42448, B = 1312.253 and C = −32.445 in the temperature range from 259.2 to 507.6 K.
| property | Type | Value [unit] | Remarks |
|---|---|---|---|
| Standard enthalpy of formation | Δ f H 0 liquid Δ f H 0 gas |
−249.4 kJ mol −1 −218.5 kJ mol −1 |
as a liquid as a gas |
| Enthalpy of combustion | Δ c H 0 gas | −1821.4 kJ mol −1 | |
| Heat capacity | c p | 125.45 J mol −1 K −1 (25 ° C) 2.16 J g −1 K −1 (25 ° C) 75.02 J mol −1 K −1 (25 ° C ) 1.29 J g −1 K −1 (25 ° C) |
as a liquid as a gas |
| Critical temperature | T c | 508.15K | |
| Critical pressure | p c | 47.582 bar | |
| Critical density | ρ c | 4.63 mol·l −1 | |
| Acentric factor | ω c | 0.30653 | |
| Enthalpy of fusion | Δ f H | 5.72 kJ mol −1 | at the melting point |
| Enthalpy of evaporation | Δ V H | 29.1 kJ mol −1 | at normal pressure boiling point |
The temperature dependence of the evaporation enthalpy can be calculated according to the equation Δ V H 0 = A e (−βT r ) (1 − T r ) β (Δ V H 0 in kJ / mol, T r = (T / T c ) reduced temperature ) with A = 46.95 kJ / mol, β = 0.2826 and T c = 508.2 K in the temperature range between 298 K and 363 K. The specific heat capacity can be calculated in the temperature range between 5 ° C and 50 ° C via a linear function with c p = 1.337 + 2.7752 · 10 −3 · T (with c p in kJ · kg −1 · K −1 and T in K) can be estimated.
Vapor pressure function of acetone
Temperature dependence of the heat of vaporization of acetone
Specific heat capacity of acetone
Safety-related parameters
Acetone forms highly flammable vapor-air mixtures. The compound has a flash point below −20 ° C. The explosion range is between 2.5 vol.% (60 g / m³) as the lower explosion limit (LEL) and 14.3 vol.% (345 g / m³) as the upper explosion limit (UEL). A correlation of the explosion limits with the vapor pressure function results in a lower explosion point of −23 ° C and an upper explosion point of 8 ° C. The explosion limits are pressure dependent. A reduction in pressure leads to a reduction in the explosion area. The lower explosion limit changes only slightly up to a pressure of 100 mbar and only increases at pressures below 100 mbar. The upper explosion limit decreases analogously with falling pressure.
| Explosion limits under reduced pressure (measured at 100 ° C) | ||||||||||||
| pressure | in mbar | 1013 | 800 | 600 | 400 | 300 | 250 | 200 | 150 | 100 | 50 | 25th |
| Lower explosion limit (LEL) | in% by volume | 2.2 | 2.2 | 2.3 | 2.3 | 2.4 | 2.4 | 2.5 | 2.6 | 2.7 | 3.6 | 5.0 |
| in g m −3 | 53 | 53 | 53 | 55 | 57 | 58 | 59 | 61 | 63 | 86 | 119 | |
| Upper explosion limit (UEL) | in% by volume | 14.3 | 14.0 | 13.7 | 13.4 | 13.2 | 13.1 | 13.1 | 13.1 | 12.5 | 10.3 | 9.0 |
| in g m −3 | 345 | 338 | 331 | 324 | 319 | 316 | 316 | 316 | 302 | 249 | 217 | |
| Maximum explosion pressure under reduced pressure | ||||||||||||
| pressure | in mbar | 1013 | 800 | 600 | 400 | 300 | 200 | 100 | ||||
| Maximum explosion pressure (in bar) | at 20 ° C | 9.3 | 7.5 | 5.5 | 3.6 | 2.7 | 1.8 | 0.8 | ||||
| at 100 ° C | 7.4 | 4.5 | ||||||||||
The maximum explosion pressure is 9.7 bar. As the temperature rises and the outlet pressure falls, the maximum explosion pressure falls. The limit gap width was determined to be 1.04 mm (50 ° C). This results in an assignment to explosion group IIA. With a minimum ignition energy of 1.15 mJ, vapor-air mixtures are extremely ignitable. The ignition temperature is 535 ° C. The substance therefore falls into temperature class T1. A sharp drop in the ignition temperature is observed under increased pressure. The electrical conductivity is rather low at 4.9 · 10 −7 S · m −1 .
| Ignition temperatures under increased pressure | ||||||||||||
| pressure | in cash | 1 | 2 | 4th | 6.8 | 16.5 | ||||||
| Ignition temperature | in ° C | 535 | 345 | 290 | 265 | 250 | ||||||
Reactions (selection)
Iodination of acetone
A special reaction here is the iodination of acetone as a classic example of pseudo-zero order reaction kinetics . Since only the enol form can be iodinated, but acetone is almost 100% ketone, the 2-propenol concentration during the reaction can be regarded as constant. Its C = C double bond reacts with iodine , splitting off an iodide ion to form a mesomeric cation, which then transfers a proton to an iodide ion.
The establishment of the keto-enol equilibrium is acid (and also base) catalyzed. The iodination is therefore greatly accelerated by the resulting hydrogen iodide ( autocatalysis ).
Iodoform reaction
When adding base, however, the iodoform reaction takes place:
Formation of dibenzalacetone
Acetone reacts in the presence of benzaldehyde in an alkaline solution to form dibenzalacetone . The reaction takes place according to the general mechanism of aldol condensation .
Also benzalaniline be synthesized - it reacts aniline with the at-in alkaline solution acetone elimination of water to Schiff base ( azomethine ). Both dibenzalacetone and benzalaniline are valuable substances because they have very reactive double bonds that can be attacked by nucleophiles .
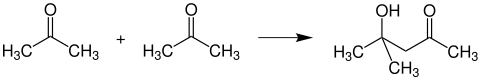
Formation of diacetone alcohol
If two acetone molecules are allowed to dimerize in an aldol-like manner under the influence of basic reagents, diacetone alcohol is formed :
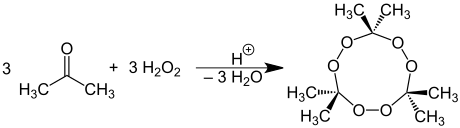
Formation of acetone peroxide
Acetone reacts with hydrogen peroxide to form acetone peroxide, which tends to detonate :
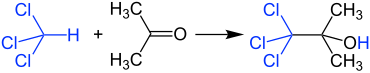
Reaction with chloroform
Acetone and chloroform must not be mixed in higher concentrations because a very violent reaction occurs in the presence of traces of alkaline substances, producing 1,1,1-trichloro-2-methyl-2-propanol . For this reason, too, chlorinated and non-chlorinated solvent waste should be collected separately in the laboratory.
use
Acetone is the starting material for numerous syntheses in the chemical industry. It is mainly used to manufacture polymethyl methacrylate (PMMA), colloquially known as acrylic glass or plexiglass. To do this, the acetone is first converted into acetone cyanohydrin by adding hydrocyanic acid , which easily splits off water in an acidic environment ( mesomeric stabilization of the double bond due to conjugation to the triple bond of the nitrile group). The resulting 2-methylpropenenitrile is converted to methyl methacrylate by adding a mixture of concentrated sulfuric acid and methanol , which is polymerized to acrylic glass in a further step.
Acetone is used industrially as a precursor for the production of diacetone alcohol by aldol addition and thus indirectly as a precursor for mesityl oxide and methyl isobutyl ketone .

Acetone is also used in small quantities as a useful solvent for resins , fats and oils, rosin , cellulose acetate, as well as nail polish remover and plastic glue. It is also used to remove contamination caused by construction foam, for example when cleaning PU foam guns. It dissolves many times its volume in ethyne (acetylene).
In some countries acetone is added in small proportions (1: 2000 - 1: 5000) to gasoline or diesel in order to achieve a more complete combustion of the fuel.
In photochemical circuit board production , acetone is used for the final degreasing of the circuit board before soldering.
Acetone-containing solutions are used in dentistry to clean prepared dentin surfaces and root canals .
biochemistry
Acetone is a ketone body formed in the liver that cannot be metabolized to any significant extent. It is therefore released through the lungs or, in exceptional cases , through the urine ( acetonuria , a symptom of diabetes mellitus ). Other ketone bodies are acetoacetic acid and 3-hydroxybutanoic acid . These can be processed in the metabolism and are involved in providing energy for the muscles.
toxicology
Acetone causes dryness on the skin as it degreases the skin. Therefore, you should grease the affected areas after contact. Inhalation of larger doses causes bronchial irritation, tiredness and headache. Very high doses have a narcotic effect .
Hexadeuteroacetone
Deuterated acetone (empirical formula: C 3 D 6 O), also called acetone-d 6 , is used as a solvent in nuclear magnetic resonance spectroscopy (NMR).
The physical properties are slightly different from the non-deuterated compound:
- Melting point: -93.8 ° C
- Boiling point: 55.5 ° C
- Density: 0.872 g / ml (25 ° C)
- Refractive index: 1.355 (20 ° C)
Web links
- Entry for Acetone in the Consumer Product Information Database
Individual evidence
- ↑ Entry on ACETONE in the CosIng database of the EU Commission, accessed on February 16, 2020.
- ↑ a b c d e f g h i j k l m n o Entry on acetone in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Peter B. Fleming, Robert E. McCarley: Chemistry of Polynuclear Metal Halides. IV. Electronic Spectra of Some Niobium and Tantalum M 6 X 12 n + Cluster Derivatives . In: Inorganic Chemistry . tape 9 , no. 6 , June 1970, p. 1347-1354 , doi : 10.1021 / ic50088a011 .
- ↑ Entry on acetone. In: Römpp Online . Georg Thieme Verlag, accessed on November 10, 2014.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Dipole Moments, pp. 9-52.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-4.
- ↑ Entry on Acetone in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 67-64-1 or acetone ), accessed on November 2, 2015.
- ↑ chemgapedia.de , learning unit acetone synthesis.
- ↑ a b I. M. Smallwood: Handbook of organic solvent properties. Arnold, London 1996, ISBN 0-340-64578-4 , pp. 27-29.
- ↑ D. Ambrose, CHS Sprake, R. Townsend: Thermodynamic Properties of Organic Oxygen Compounds. XXXIII. The Vapor Pressure of Acetone. In: J. Chem. Thermodyn. 6, 1974, pp. 693-700, doi: 10.1016 / 0021-9614 (74) 90119-0 .
- ↑ a b K. B. Wiberg, LS Crocker, KM Morgan: Thermochemical Studies of Carbonyl Compounds. 5. Enthalpies of Reduction of Carbonyl Groups. In: J. Am. Chem. Soc. 113, 1991, pp. 3447-3450, doi: 10.1021 / ja00009a033 .
- ^ CB Miles, H. Hunt: Heats of Combustion. I. The Heat of Combustion of Acetone. In: J. Phys. Chem. 45, 1941, pp. 1346-1359; doi: 10.1021 / j150414a002 .
- ↑ a b c R. Malhotra, LA Woolf: Thermodynamic Properties of Propanone (Acetone) at Temperatures from 278 K to 323 K and Pressures up to 400 Mpa. In: J. Chem. Thermodyn. 23, 1991, pp. 867-876, doi: 10.1016 / S0021-9614 (05) 80282-4 .
- ↑ a b J. Chao: Thermodynamic Properties of Key Organic Oxygen Compounds in the Carbon Range C 1 to C 4 . Part 2. Ideal Gas Properties. In: J. Phys. Chem. Ref. Data . 15, 1986, pp. 1369-1436, doi: 10.1063 / 1.555769 .
- ↑ a b c A. N. Campbell, RM Chatterjee: The critical constants and orthobaric densities of acetone, chloroform benzene, and carbon tetrachloride . In: Canadian Journal of Chemistry . 47, 1969, pp. 3893-3898, doi : 10.1139 / v69-646 .
- ↑ J. Schmidt: Design of safety valves for multi-purpose systems according to ISO 4126-10. In: Chem. Ing. Techn. 83, 2011, pp. 796-812, doi: 10.1002 / cite.201000202 .
- ↑ KK Kelley: The heats capacities of isopropyl alcohol and acetone from 16 to 298 ° K and the corresponding entropies and free energies. In: J. Am. Chem. Soc. 51, 1929, pp. 1145-1150, doi: 10.1021 / ja01379a022 .
- ^ A b V. Majer, V. Svoboda: Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation. Blackwell Scientific Publications, Oxford 1985, p. 300.
- ↑ a b c d e E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ↑ a b c d D. Pawel, E. Brandes: Final report on the research project, the dependence of safety parameters on the pressure below atmospheric pressure. ( Memento of December 2, 2013 in the Internet Archive ), Physikalisch-Technische Bundesanstalt (PTB), Braunschweig 1998.
- ↑ JB Fenn: Lean Flammability Limit and Minimum Spark Ignition Energy. Commercial Fluids and Pure Hydrocarbons. In: Ind. Eng. Chem. 43, 1951, pp. 2865-2869; doi: 10.1021 / ie50504a057 .
- ↑ HF Calcote, CA Gregory, CM Barnett, RB Gilmer: Spark Ignition - Effect of Molecular Structure. In: Ind. Eng. Chem. 44, 1952, pp. 2656-2662; doi: 10.1021 / ie50515a048 .
- ↑ Technical rule for hazardous substances TRGS 727, BG RCI leaflet T033 Avoidance of ignition hazards due to electrostatic charges , status August 2016, Jedermann-Verlag Heidelberg, ISBN 978-3-86825-103-6 .
- ^ FA Carey, RJ Sundberg: Organic Chemistry. Wiley-VCH Verlag, 2004, ISBN 3-527-29217-9 .
- ↑ Lutz Roth, Ursula Weller: Hazardous Chemical Reactions , ISBN 3-609-73090-0 , ecomed security; 2005.
- ↑ External identifiers of or database links for Deuterated Acetone : CAS number: 666-52-4, EC number: 211-563-9, ECHA InfoCard: 100.010.514 , PubChem : 522220 , ChemSpider : 455535 , Wikidata : Q1032873 .
- ↑ Acetone-d6 data sheet from Sigma-Aldrich , accessed on August 31, 2019 ( PDF ).
- ↑ Compared to 0.791 g / ml (25 ° C) for the nondeuterated compound. Acetone data sheet from Sigma-Aldrich , accessed on August 31, 2019 ( PDF ).