Babesiosis of the dog
The babesiosis of the dog ( synonym "dog malaria," piroplasmosis ) is a by protozoa of the genus Babesia caused infectious disease in dogs , the destruction of the red blood cells and therefore a more or less pronounced anemia ( anemia causes). The disease is usually acute with a high fever and, without treatment, ends fatally within a few days. It is transmitted by ticks . While babesiosis was primarily a "travel sickness" until the 1970s, it now occurs naturally north of the Alps due to the expansion of the range of the alluvial forest tick . The diagnosis is confirmed by evidence of the Babesia DNA or a microscopic examination of the blood. Antiprotozoal drugs are used for treatment .
Other animal species or humans are not endangered by the babesia, which can cause disease in dogs. However, diseases due to mostly host-specific babesias also occur in other mammals (→ babesiosis in humans and systematics of babesia ).
Pathogen and Spread
Babesias are unicellular organisms that parasitize the red blood cells. They are assigned to the apicomplexa . A section of their reproduction cycle takes place in the intermediate host - different types of ticks . Dog babesiosis is caused by several species of babesia that are not pathogenic to other animal species . In one study, antibodies against Babesia canis were found in horses, but the infection in these animals has no clinical symptoms and is self-limiting.
Babesia canis (Piana & Galli-Valerio, 1895) is a relatively large Babesia species (2–4 × 4–7 μm) that occurs worldwide. Today there are three subspecies that differ in terms of their DNA and their vector , but not morphologically:
- Babesia canis canis is transmitted by the alluvial forest tick ( Dermacentor reticularis ), which has now spread throughout Central Europe. This Babesia subspecies is most frequently responsible for diseases in dogs in German-speaking countries. Originally only occurring in North Africa, North and Central Italy, France and the southern part of Hungary and Austria (" Mediterranean disease "), there are now natural herds in Germany, Switzerland, Holland and Poland. The pathogen is very disease-causing . There are two different tribes. The French tribe comes from the northern and eastern Mediterranean and is now also found in some south-western regions of Germany. The Hungarian tribe occurs mainly in the Balkans and the Ukraine, but now also in some regions of Eastern Germany.
- Babesia canis vogeli is transmitted by the brown dog tick ( Rhipicephalus sanguineus ). Infections with this pathogen are rare in Central Europe and are mild. Is spread c example. vogeli in North Africa, the Mediterranean and France.
- Babesia canis rossi is distributed by Haemaphysalis elliptica and occurs only in sub- Saharan Africa . The pathogen is the Babesia species that causes the most disease.
In more recent studies, these three large babesia species are also counted as separate species, and there are two other isolates (North Carolina isolate and Great Britain isolate) that indicate other large babesia species.
Babesia gibsoni (Patton, 1910) is another species of Babesia found in dogs. It is smaller (1.1–2 × 1.2–4 μm) and can therefore be distinguished morphologically from B. canis . The pathogen is spread mainly in Asia and the United States, a distinction is an Asia - and California - genotype . It is transmitted by ticks of the genera Haemaphysalis ( Haemaphysalis spinosa ) and Rhipicephalus . In 2007, two local infections with the Asian genotype were described in Germany for the first time. A current study proposesclassifyingthe “little Babesia” of the California genotype as a separate species, Babesia conradae . The species “ Babesia vulpes ” (formerly Theileria annae ),which is also one of the “small Babesia”, primarily attacks foxes and occurs in the Pyrenees and North America. It is probably transmitted by the hedgehog tick ( Ixodes hexagonus ).
The first evidence of the disease was made in the United States in 1934. But there were already reports of dog diseases in Africa from 1896, which indicate babesiosis. The original distribution area of babesiosis ( enzootic area ) within Europe was limited to southern Europe until the 1970s, so that the disease in Germany occurred almost exclusively in dogs after vacation trips to this region. With the spread of the alluvial forest tick throughout Central Europe, local cases of illness also occur in Germany: Around a third of the sick dogs have never been abroad. Babesia contamination of alluvial forest ticks is still relatively low in Germany, but is steadily increasing. About 0.5% of the alluvial forest ticks are babesia carriers. After local infections were initially only observed in the Upper Rhine , there are now enzootic areas in Saarland , Rhineland-Palatinate , in the Isar floodplains near Munich, in the vicinity of Regensburg , in the Elbe meadows and in Brandenburg . Several thousand diseases are currently diagnosed in Germany each year. Of these, around 300–400 are local infections, almost all of which occur in Saarland and the Upper Rhine.
Disease emergence
The transmission at the tick bite takes about 48 to 72 hours, under experimental conditions of a transmission could by attachment of the tick already after 12 hours , c. canis can be detected. When the tick attaches to the host, the sporozoites, which are dormant in various organs, are activated by irritation of the nervous system and develop into kinetes, which then migrate into the salivary glands and enter the dog's bloodstream with the tick's saliva. In addition to being transmitted by ticks, infection from dog to dog is possible via a blood transfusion or through blood-blood contact - for example, when biting. A transmission from the bitch to her offspring ( “vertical infection” ) is suspected and has been proven for B. gibsoni .
The sporozoites penetrate the red blood cells ( erythrocytes ) of the dogs and carry out an asexual multiplication phase ( merogony ). The developmental stages that arise (so-called merozoites ) lead to damage to the erythrocytes, are released after they have been destroyed and can then penetrate into new, as yet unaffected erythrocytes. In response to the infection, the organism initially shows an acute phase reaction with an increase in C-reactive protein and fibrinogen , a drop in platelets and leukocytes and a drop in blood pressure . In the course there is an immune response with formation of IgG - and IgM - antibodies . A complete elimination of the pathogen by the dog's immune system does not take place, however, so that these animals represent a constant source of infection (pathogen reservoir ) and thus ensure that the infection cycle is maintained.
Ticks pick up the infected erythrocytes when they suckle. In the tick intestine, the merozoites develop into sexual baby stages (gamonts and gametes). These differentiate into kinetes, which penetrate the eggs within the tick's ovaries and thus pass the pathogen on to the offspring of the tick (transovarian transmission). This transovarian transmission means that not only adult ticks but also nymphs are carriers of Babesia. In addition, the kinetes migrate into the tick's salivary glands, where they differentiate into the sporozoites, which are infectious for dogs.
Clinical picture
In Germany, the most acute form of Babesia canis canis infection occurs. The incubation period is 5 to 7 days, rarely it can last up to three weeks after the tick bite. Signs of illness ( symptoms ) are a reduced general condition and fever , followed by loss of appetite , weight loss and fatigue . One or two days later, due to the breakdown of the red blood cells ( hemolysis ), anemia , blood urine , excretion of the blood pigment breakdown product bilirubin in the urine ( bilirubinuria ) and possibly also jaundice . A liver - and spleen enlargement is common. In severe cases, ascites and fluid retention ( edema ) as well as skin and mucous membrane bleeding due to a lack of blood platelets ( thrombocytopenia ) and blood clotting within the blood vessels ( disseminated intravascular coagulopathy ) occur. Inflammation of the mouth ( stomatitis ) and stomach lining ( gastritis ) as well as the muscles ( myositis ) are common. A central nervous form with epilepsy-like seizures , movement disorders and paralysis is also possible. If left untreated, the acute form ends within a few days with death from shortness of breath , anemia and kidney failure , which is a dreaded complication of babesiosis. The rare peracute course ends fatally within one to two days without clear symptoms. The infection with B. canis rossi is similar to that with B. canis canis .
The degree of clinical manifestations depends on various factors. In the classic natural herds of Babesia canis canis (southern Austria, Hungary, northern Italy), the young animals are generally protected by antibodies from the first milk ( colostrum ) of the bitch due to a high level of contamination , develop extensive protection through primary latency and become immune vectors. The chronic or subclinical course of the disease with unspecific symptoms such as intermittent fever , unwillingness to eat, anemia and general weakness dominates here. Infections with B. canis vogeli and the “little Babesia” are also milder.
Diagnosis
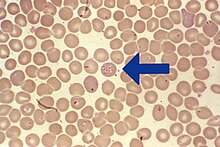
Babesiosis can clinically be confused with a variety of other febrile illnesses. The diagnosis can be made using a normal blood smear (“thin drop”) or the so-called “thick drop” , whereby capillary blood is more sensitive than venous blood. The pathogens can only occur in small numbers in the early phase of the infection and in the phases between the multiplication spurts in the blood ( parasitemia ) and can therefore be overlooked. Evidence in the blood smear is only certain about seven days after infection. The Babesia can be detected under the microscope , whereby the Giemsa staining - in contrast to the usual rapid staining - is the most reliable. B. canis appears as pear-shaped structures arranged in pairs or in larger groups in rosettes in the red blood cells, B. gibsoni as ring-shaped structures. A safe PCR -Proof the DNA of the pathogen is already 3 to 5 days after infection possible.
Serological tests such as the immunofluorescent antibody test and the enzyme-linked immunosorbent assay (ELISA) are of no importance in the acute course, as the animals have not yet formed any antibodies . Antibodies are detectable no earlier than 10 days after infection. With a chronic course, cyclical changes in the antibody level occur.
If the leukocyte count <7250 / µl, the platelet count <55,000 / µl and the reticulocyte count <61,600 / µl during a blood test, babesiosis should always be considered with a corresponding preliminary report, so that direct pathogen detection should be attempted. In the differential diagnosis, anaplasmosis , immune-related hemolytic anemia , immune-related thrombocytopenia , infection with Mycoplasma haemocanis , inflammation of the urinary tract and poisoning with onions must be excluded.
Treatment and prevention
Since the disease can quickly become fatal without treatment, therapy should be initiated immediately if it is suspected. Antiprotozoic drugs such as imidocarb or diminazen are good against B. canis , but not very effective against "small babesia". Imidocarb can also be administered once for prophylaxis when traveling to endemic areas - the protection lasts for about three weeks. A combination of atovaquone and azithromycin can also cure chronic B. gibsoni infections . Phenamidine is also effective against “little babesias”, but is currently not available in Germany. In acute cases, if the hematocrit is below 20, a blood transfusion or the administration of hemoglobin-glutamer 200 is indicated. Treatment with imidocarb is handled differently depending on the region. In the original endemic areas, it is used only once at a low dose to suppress the acute illness, but not to completely eliminate the pathogen in order to develop a long-term, resilient immunity. In non-endemic regions, however, the active ingredient is applied twice in higher doses. This fights the pathogen completely, but the subsequent immunity only lasts for 1 to 2 years.
The most important prophylaxis is to search the animal for ticks after every walk and to remove them immediately. Protection against ticks by externally applied tick-killing agents ( acaricides such as Amitraz , Deltamethrin , Fipronil , Flumethrin or Permethrin ) or oral acaricides such as Fluralaner or Afoxolaner is useful, as they also reduce the risk of other diseases such as borreliosis which can be transmitted to dogs by ticks , Lower Ehrlichiosis , Hepatozoonosis or TBE .
Against B. c. canis and B. c. rossi there is a vaccine (trade name Nobivac Piro ) that does not protect against infection, but significantly alleviates the disease. Despite EU approval, the vaccine is not available in Germany, but is available in Switzerland, Austria and France. It must be administered every six months after two basic vaccinations and should not be administered together with other vaccinations or to animals that have already been infected. The main side effects observed are swelling at the injection site, fever, fatigue and a stiff gait, which subside on their own. The Standing Vaccination Commission in the Federal Association of Practicing Veterinarians does not currently recommend general use and counts them among the not absolutely necessary ( non-core ) vaccinations.
literature
- Dieter Barutzki et al .: The babesiosis of the dog . In: Deutsches Tierärzteblatt . 55, 2007, ISSN 0340-1898 , pp. 284-293.
- Katrin Hartmann: Parasitic Infections . In: Peter F. Suter and Hans G. Nobody (eds.): Internship at the dog clinic . 10th edition. Paul-Parey-Verlag, Stuttgart 2006, ISBN 3-8304-4141-X , pp. 316-324.
- Cornelia Heile and Eberhard Schein (eds.): Guideline for preventing the transmission of pathogens through blood-sucking vectors in dogs . Federal Association of Practicing Veterinarians, 2007.
- Maja Hirsch: Babesiosis . In: Travel sickness in Europe. IDEXX Laboratories 2009, pp. 2-5.
- Individual evidence
- ↑ S. Hornok et al .: Serological evidence for Babesia canis infection of horses and of endemic focus of B. caballi in Hungary. In: Acta Vet Hung. 55 (2007), pp. 491-500. PMID 18277708
- ↑ Hans Dautel et al .: Evidence for an increased geographical distribution of Dermacentor reticulatus in Germany and detection of Rickettsia sp. RpA4 . In: International Journal of Medical Microbiology 296 (2006), Supplement 1, pp. 149-156. PMID 16524777
- ↑ a b Barbara Hinney and Michael Leschnik: Travel parasitoses of dogs and cats . In: Small Animal Practice . tape 60 , no. 5 , 2015, p. 254-282 .
- ↑ a b Nico Pantchev: Tick-Borne Travel infections in dogs: ehrlichiosis and babesiosis. In: Veterinärspiegel 4 (2012), pp. 162–170.
- ^ AJ Birkenheuer: Geographic distribution of babesiosis among dogs in the United States and association with dog bites: 150 cases (2000-2003). In: J Am Vet Med Assoc. 227 (2005), pp. 942-947. PMID 16190594
- ↑ A. Matsuu et al .: Incidence of canine Babesia gibsoni infection and subclinical infection among Tosa dogs in Aomori Prefecture, Japan. In: J Vet Med Sci. 66 (2004), pp. 893-897. PMID 15353837
- ↑ K. Hartelt et al .: First evidence of Babesia gibsoni (Asian genotype) in dogs in Western Europe. Vector Borne Zoonotic Dis. 2007 Summer; 7 (2): 163-166. PMID 17627433
- ↑ AM Kjemtrup et al .: Babesia conradae, sp. Nov., a small canine Babesia identified in California. Vet Parasitol. 2006 May 31; 138 (1-2): 103-111. PMID 16524663
- ↑ Gad Baneth, corresponding author Monica Florin-Christensen, Luís Cardoso, and Leonhard Schnittger: Reclassification of Theileria annae as Babesia vulpes sp. nov. In: Parasite Vectors. 207 (2015); 8 PMC 4393874 (free full text)
- ↑ a b T. J. Naucke: Babesiose / Piroplasmose - an update. In: Veterinärspiegel 1 (2008), pp. 14-14.
- ↑ Dog malaria on the rise again in 2008
- ↑ S. Fukumoto et al .: Fatal experimental transplacental Babesia gibsoni infections in dogs. Int J Parasitol. 35 (2005), pp. 1031-1035. PMID 15979628
- ↑ TP Schetters et al .: Systemic inflammatory responses in dogs experimentally infected with Babesia canis; a haematological study. In: Vet Parasitol. 162 (2009), pp. 7-15. PMID 19269099
- ↑ a b M. Böhm et al .: Capillary and venous Babesia canis rossi parasitaemias and their association with outcome of infection and circulatory compromise. In: Vet Parasitol. 141 (2006), pp. 18-29. PMID 16806713
- ↑ L. Solano-Gallego et al .: Babesia canis canis and Babesia canis vogeli clinicopathological findings and DNA detection by means of PCR-RFLP in blood from Italian dogs suspected of tick-borne disease. In: Veterinary Parasitology 157 (2008), pp. 211-221. PMID 18789581
- ^ HJ Vial and A. Gorenflot: Chemotherapy against babesiosis. In: Vet Parasitol. 138 (2006), pp. 147-160. PMID 16504402
- ^ AB Zambelli and AL Leisewitz: A prospective, randomized comparison of Oxyglobin (HB-200) and packed red blood cell transfusion for canine babesiosis . In: Journal of Veterinary Emergency and Critical Care 19 (2009), pp. 102-112.
- ↑ Laboklin currently 03/2016
- ↑ vetpharm.uzh.ch
- ↑ Vaccination recommendations of the Standing Vaccination Commission of the bpt (PDF; 590 kB)