Chlamydia
| Chlamydiaceae | ||||||||
|---|---|---|---|---|---|---|---|---|

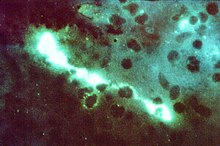
Chlamydia trachomatis , inclusion bodies (brown) in a McCoy cell culture |
||||||||
| Systematics | ||||||||
|
||||||||
| Scientific name | ||||||||
| Chlamydiaceae | ||||||||
| Rake 1957 (Approved Lists 1980) emend. Everett et al. 1999 | ||||||||
| Genera | ||||||||
|
Chlamydia is a common name in everyday language for species within the Chlamydiaceae family . These are very small, gram-negative bacteria that, as parasites, can only multiply within a host cell . They can infect a wide variety of living things, including humans. An infection with chlamydia is known as chlamydiosis . The diseases concern u. a. the mucous membranes in the eyes, respiratory tract and genital area.
The representatives of the Chlamydiaceae go through two forms in their development cycle, as infectious elementary bodies and as reticular bodies, which represent the vegetative form. They cannot be grown on conventional culture media . In the past, all known species were assigned to the genus Chlamydia , as a result of genetic investigations, the systematics of the order Chlamydiales, the family, the genera and the species were updated in 1999 . Since then, two genera - Chlamydia and Chlamydophila - have belonged to the Chlamydiaceae family . However, this system is controversial.
features
Appearance
The representatives of the Chlamydiaceae can only intracellularly, i.e. H. multiply within the cells of a host . The Chlamydia exist in the form of metabolically active elementary bodies (EK) - in English elementary bodies , EB - from 0.2 to 0.4 micrometers (microns) in diameter, for the infection are important. After an infection, 0.6–1.5 µm large reticulate bodies ( reticular bodies , abbreviation RK) are found in the host cells. They represent the multiplying, metabolically active form. In the English specialist literature they are referred to as reticulate bodies (RB), in the German, v. a. medical literature, the terms initial bodies and inclusion bodies can also be found .
The representatives of the Chlamydiaceae are called Gram- negative bacteria because they - as elementary bodies - are colored red in the Gram stain by the dyes used. This is usually caused by a thin layer of murein (consisting of peptidoglycans) in the cell wall , in contrast to the gram-positive bacteria, which have a thick layer of murein. Genetic studies have shown that the members of the Chlamydiaceae family have genes that are required for the synthesis of peptidoglycans. However, the studies show that their cell walls contain only very small amounts or no peptidoglycan at all.
Growth and metabolism
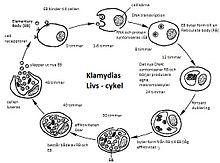
Chlamydia go through two forms in their development cycle: Outside their host cells they exist as elementary bodies (EK). The EK has no metabolic activity , but depending on the type of bacteria it is quite resistant to environmental influences (e.g. osmotic changes and dehydration) and can therefore survive outside the host organism. The host cells are infected by the elementary bodies. Once absorbed into the cell, the elementary bodies transform into reticular bodies (RK), which have an active metabolism and multiply within the host cell.
The elementary body must come into contact with the host cell, these are epithelial cells . It attaches to their membrane and is absorbed by the cell in a kind of bag (a membrane inclusion called an endosome ), a process known as endocytosis . The EK enclosed in the endosome transform into reticular bodies (RC) within one to two hours. The endosomes do not fuse with lysosomes , as actually happens in the process of phagocytosis . Research results from 2006 suggest that the reticular bodies succeed in incorporating lipids of the host cell into their envelope membrane and thus prevent the lysosomes of the host cell from recognizing them as foreign bodies and from merging with them. Since the RK are not destroyed, they multiply within the endosome through cell division and are dependent on metabolic products and ATP from the host cell for growth. The generation time is 2-3 hours. Their accumulation in the endosome leads to the designation as inclusion or inclusion bodies . Then they transform back into elementary bodies. A few days after the infection, the host cell is destroyed by lysis and the elementary bodies are released again, they can now infect other cells. It has also been observed that the EKs are released without destroying the cells, as the endosomes fuse with the cell membrane and release their content - the elementary bodies - to the outside.
In addition to these two main forms of the development cycle, another form is known, the aberrant corpuscles . This is an intracellular, persistent form that arises under non-optimal growth conditions of the host cells. It is viewed as a permanent form with a reduced metabolism and can develop back into elementary bodies. The aberrant bodies are also of medical importance as their presence has been linked to reactive arthritis .
Because of the parasitic metabolism, chlamydia cannot be cultivated on the nutrient media commonly used in microbiology ; cell cultures are required instead . Usually HeLa cells (human epithelial cells ) are used, the human Hep2 and McCoy cell lines are also used.
The representatives of the Chlamydiaceae have lipopolysaccharides deposited on the cell wall . These compounds of fat-like components, combined with three sugar components , act as antigen and can be used serologically for the detection. The sugar building blocks present in the core region of the lipopolysaccharide are 2-keto-3-deoxy-octonate (KDO), the three monosaccharides are linked with a glycosidic bond as follows : α-KDO- (2 → 8) -α- KDO- (2 → 4) α-KDO.
To distinguish between Chlamydia and Chlamydophila species, it is important to know that only the former can produce glycogen as a storage substance. In Chlamydia species, the glycogen can be detected in the reticular bodies by means of an iodine-potassium iodide solution (Lugol's solution) and stains these inclusion bodies brown.
Some strains of Chlamydophila psittaci and Chlamydia trachomatis require ATP ( adenosine triphosphate ) for growth , which is supplied to them by the host cell, as they cannot synthesize it themselves. ATP is the universal energy carrier in every cell , so the bacteria were seen as energy parasites. However, genetic studies on a strain of Chlamydia trachomatis have shown that it has genes that are required for ATP synthesis . At the moment (2013) it has not yet been conclusively clarified whether all representatives of the Chlamydiaceae are actually dependent on ATP from the host cells.
The osmotic stability of the elementary bodies is maintained by an envelope protein . It consists of several protein subunits linked by disulfide bridges . One component is called MOMP (abbreviation for major outer-membrane protein (English), translated as the main protein of the outer membrane ). This protein has a molecular mass of about 40 kDa ( kilodaltons ). In addition, there is a hydrophilic , cysteine- rich protein with a molecular weight of about 60 kDa and a small cysteine-rich lipoprotein . When the elementary body infects a host cell, the disulfide bridges within the protein subunit and also between them are chemically reduced, similar to the reduction of cystine . This enables the conversion of the elementary body (EK) into the reticular body (RK).
genetics
The cells contain the nucleic acids DNA and RNA , which is important because until around 1970 it was assumed that these organisms, known in medicine as pathogens , are viruses . The genome of several bacterial strains from the Chlamydiaceae family has already been completely sequenced . The size of the genome of all representatives examined so far is between 1000 and 1400 kilobase pairs (kb), which is only 25% of the genome size of Escherichia coli . The small size of the genome is an indication of the parasitic way of life; the chlamydia have lost the ability to synthesize numerous metabolites over the course of time , as they are obtained by the host cells.
The result of the sequencing shows a GC content (the proportion of the nucleobases guanine and cytosine ) in the bacterial DNA of around 40 mol percent. Another characteristic is that there are either one or two ribosomal operons in the genome. An operon is actually a functional unit of DNA, but in this case the operon is found in ribosomal RNA . Many representatives (for example Chlamydophila felis ) have a plasmid with a genome size of about 7.5 kb in addition to the bacterial chromosome .
Pathogenicity
The representatives of the Chlamydiaceae are all classified as pathogenic (disease-causing) because they cause various infectious diseases. Several species are identified as zoonotic pathogens because an infection can be transmitted directly or indirectly between animals and humans. The exact allocation of a species to a risk group is made by the Biological Agents Ordinance in conjunction with the TRBA ( Technical Rules for Biological Agents) 466.
proof
Cultural evidence is a reliable method for diagnosing chlamydia, but is not used in routine diagnostics. The representatives of the Chlamydiaceae cannot be cultivated on the nutrient media commonly used in microbiology; instead, cell cultures are required. This is mainly done by special laboratories, with the species Chlamydophila psittaci there is the additional difficulty that cultivation is only permitted in laboratories of protection level 3 . The detection takes place with the help of fluorescence microscopy .
Serological evidence
The lipopolysaccharides deposited on the cell wall act as antigen and can be used serologically for detection in the context of the antigen-antibody reaction . In clinical diagnostics, however, detection is more common by means of an increased titer of antibodies ; the antigen used is usually not species-specific and consequently indicates several Chlamydophila or Chlamydia species. Furthermore, the detection of the antibodies does not clearly distinguish between healed and persistent infections.
The detection of specific antibodies in the serum is carried out in the routine laboratory for Chlamydophila pneumoniae and C. psittaci with the ELISA method. Here, too, the restriction applies that only genus-specific antibodies are usually detected.
Further serological methods compare the reactivity of the antibodies in the patient's serum with antigens from Chlamydia trachomatis , Chlamydophila pneumoniae and Chlamydophila psittaci . The antigen with the strongest reactivity indicates the specificity of the antibodies. The microimmunofluorescence test (MIF) and an immunoblot (Western blot) are based on this. For this, recombinant (genetically engineered) antigens are required. There are commercial offers for both methods.
Immunofluorescence is often used . It is based on the antigen detection, the antibody used is a fluorescent dye labeled . A smear containing the bacteria is used as the test material. Again, false positive results can occur due to non-species-specific antibodies, which should be confirmed with a second method. Both the low sensitivity and the low specificity of these methods allow the detection of chlamydia only with a high bacterial density. Rapid tests based on this principle do not provide reliable results.
Antigen detection methods (in addition to direct immunofluorescence, also the ELISA method) are often used in veterinary medicine despite their low specificity - for example for the detection of Chlamydophila psittaci - because the samples usually contain many bacteria in the presence of an infection. These methods have not been validated for diagnosis in humans and are therefore not recommended.
Molecular biological evidence
The detection of certain parts of the bacterial genome using the PCR method ( polymerase chain reaction ) is much more specific . In this process, gene segments that are typical for the type of bacteria are duplicated ( amplified ) and detected.
In the context of diagnostics, so-called nucleic acid amplification techniques (NAT) based thereon are used, which have a high level of sensitivity and specificity. For example, when C. trachomatis is detected, a DNA sequence is amplified from the plasmid. Since the plasmid occurs in ten copies in a cell, a high level of sensitivity is achieved. However, it must be checked regularly whether the target sequence is actually still present in the bacterium to be detected, otherwise false negative NAT results will occur. A different NAT protocol is used for this purpose, i.e. a procedure with a different target sequence typical for the species. The MOMP gene is suitable for C. trachomatis . Since the nucleic acid amplification technique has a high sensitivity, clinical samples such as smears, respiratory samples or urine can be examined directly with it.
For the detection of C. trachomatis , the World Health Organization (WHO) evaluated the known data using various test methods: Urine or genital fluid were used as samples. The nucleic acid amplification techniques show the most reliable results with about 87% sensitivity and 99.2–99.8% specificity.
No commercial PCR protocol is available for the detection of C. psittaci and C. pneumoniae so that this method is reserved for special laboratories. A method developed in 2010 is based on Real Time Quantitative PCR ( q-PCR ), using the fluorescent dye SYBR Green , which attaches to the gene segments to be detected and causes fluorescence. The strength of the fluorescence is recorded in real time during a PCR cycle (hence the term real time ) and is used for the quantitative determination of the gene segments present and thus a quantitative recording of the bacterial species. The method developed in Japan aims at the detection of C. psittaci , but other animal pathogenic Chlamydophila species, u. a. C. felis to prove it. Clinical samples (e.g. faeces ) or infected cells can be used, from which the DNA is first extracted and then used for the q-PCR .
Occurrence
Occurrence of Chlamydia
Chlamydia trachomatis occurs only in humans as a pathogen. C. muridarum (considered a variant of C. trachomatis before 1999 ) occurs in representatives of the Muridae (mouse-like species), for example in mice and hamsters . Chlamydia suis is found in pigs, the natural host is the wild boar , but it is also found in domestic pigs . C. felis (formerly Chlamydophila felis ) is endemic in domestic cats , the pathogen can be detected worldwide. Chlamydia abortus (formerly Chlamydophila abortus ) is endemic to ruminants such as cattle, sheep and goats. In individual cases it was also detected in horses, rabbits , guinea pigs and mice, and also in individual cases in humans through a zoonosis.
Occurrence of Chlamydophila
Chlamydophila pneumoniae occurs almost exclusively in humans, the pathogen can be detected worldwide. Biovars were found sporadically in animals, these were horses and koalas , a connection with human diseases has not yet been shown. The natural reservoir of C. psittaci is birds, especially parrots . In 2012, a genetic investigation of the known genotypes of Chlamydophila psittaci was carried out , these were isolated from the following birds: cockatoos , budgies , loris and other parrots, pigeons , ducks , geese , turkeys and ratites . Some strains have also been found in humans (as a result of ornithosis ), domestic cattle and muskrats .
C. pecorum can be found in various mammals without a specific host being recognized. It was found in ruminants ( cattle , sheep and goats ), koalas and pigs. C. caviae occurs in guinea pigs, especially in representatives of the genus Cavia (real guinea pigs), to which the house guinea pig also belongs. It is believed to be specific to this genus because attempts to infect other related rodents (including hamsters and gerbils ) have failed, with one exception.
Occurrence of further representatives of the order
The species Simkania negevensis, which belongs to the order Chlamydiales , also occurs as a pathogen in humans, but it has also been detected in free-living amoebas . This is typical for the Parachlamydiaceae family, which also belongs to this order; they are found almost exclusively in amoebas.
Systematics
The name chlamydia is derived from the Greek , χλαμύς ( chlamys , genitive chlamydos ) denotes a short coat, see also " Chlamys (coat) ".
Due to their small size and their purely intracellular replication, chlamydia was classified as a virus until the 1960s . In 1966 they were recognized as a separate order Chlamydiales of bacteria. On the basis of more recent 16S rRNA analyzes, they are now placed in the Chlamydiae department. Further investigations have shown a close relationship between the Chlamydiae and the departments of Verrucomicrobia and Planctomycetes . Based on these investigations, these three departments form the so-called PVC superphylum.
According to the current system , the order Chlamydiales is divided into four families:
- Chlamydiaceae Rake 1957
- Parachlamydiaceae Everett et al. 1999
- Simkaniaceae Everett et al. 1999
- Waddliaceae Rurangirwa et al. 1999
Before 1999, all known representatives of the family Chlamydiaceae were assigned, which were referred to in the medical field as Chlamydia, the common characteristic was the parasitic way of life.
This morphologically oriented system in microbiology is supported by chemotaxonomic characteristics and biochemical or metabolic physiological properties. Since genetic investigations by sequencing the DNA ( deoxyribonucleic acid ) and RNA ( ribonucleic acid ) have been possible, these have been used to elucidate the tribal history and the relationships between the organisms. In addition to the DNA sequences of the genome, especially in bacteria, the 16S rRNA is also examined, a typical representative of ribosomal RNA for prokaryotes . Genetic studies of the DNA sequences are carried out e.g. B. by means of DNA-DNA hybridization .
The investigations of Everett u. a. you got a more precise picture of the relationships between the chlamydia. Among other things, the sequences of the 16S and 23S rRNA were examined and compared with existing data as part of a phylogenetic analysis, with which a phylogenetic tree can then be created. In addition, criteria were set up with which one can check whether organisms that are not yet clearly defined in taxonomy are members of the Chlamydiaceae family. For this purpose, the strain B577 is defined as a typical example of the family. An isolate must match the 16S rRNA genes of strain B577 more than 90% in order to be recognized as a member of the family.
The results of Everett, Andersen and Bush - especially the resulting new systematic - were critically evaluated by experts. In addition to doubts about the criteria for taxonomic classification, the main concern expressed in 2001 was that the new names Chlamydia and Chlamydophila could lead to confusion among health care professionals . The background is that the pathogens cause diseases worldwide and therefore the term chlamydia or chlamydia could only be established over a longer period of time. However, the new taxonomy is validly published according to the criteria of the bacterial systematics . Everett and Andersen, in a reply to the critics, pointed out that important sequence databases (such as GenBank ) and collections of microorganisms (such as the ATCC ) have already adopted the names. Since then, the term chlamydia (or chlamydiae in English) has been used, especially in the medical field, both for the family Chlamydiaceae and for other representatives in the order Chlamydiales, in some cases the outdated generic name Chlamydia for species can still be found in the microbiological specialist literature of the genus Chlamydophila .
In 2009 some scientists suggested that the former generic name Chlamydia should be used again for all species. The reason given was that the results of the 16S rRNA sequence analyzes were not compatible with the natural evolutionary history. So far (as of 2018), according to the International Code of Nomenclature of Bacteria , both generic names - Chlamydophila and Chlamydia - are still valid taxa, corresponding to that of Everett et al. a. The known species are assigned to the newly introduced systematics, but some Chlamydophila have already been reclassified as Chlamydia species. In 2009 the International Commission for the Systematics of Prokaryotes set up a subcommittee on the taxonomy of the Chlamydiae to deal with this controversial topic. In 2010 a working group was set up by this subcommittee. Your task is to check all the data that result from the genome sequencing available to date in order to determine whether the genetic deviations are sufficient for the establishment of two genera. With regard to the criteria for belonging to the Chlamydiaceae family, the subcommittee confirmed in 2011 that the phylogenetic analysis of the sequences of the 16S and 23S rRNA is suitable. In addition, other genes (English core genes , "core genes") should also be tested, which reveal differences more than the genes of the ribosomal RNA. Another push came in 2015 by a group of scientists who again doubted that the phylogenetic studies justify the split in two ways.
The following cladogram represents a hypothesis of the relationships within the order Chlamydiales (status 2001):
| NN |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
The Chlamydiaceae family includes the two genera Chlamydia and Chlamydophila , which contain numerous species that are pathogenic to humans and animals. Chlamydia trachomatis and Chlamydophila psittaci are each the type species . Currently (as of 2018) the following species of the two genera are known:
-
Chlamydia
Jones et al. 1945 (Approved Lists 1980) emend. Everett et al. 1999
- Chlamydia abortus ( Everett et al. 1999) Sachse et al. 2015, comb. nov. (previously Chlamydophila abortus Everett et al. 1999, sp. nov. [as basonym ], before that a variant of Chlamydia psittaci )
- Chlamydia avium Sachse et al. 2015, sp. nov.
- Chlamydia felis ( Everett et al. 1999) Sachse et al. 2015, comb. nov. (previously Chlamydophila felis Everett et al. 1999, sp. nov. [as basonym ], before that a variant of Chlamydia psittaci )
- Chlamydia gallinacea Sachse et al. 2015, sp. nov.
- Chlamydia muridarum Everett et al. 1999 (previously a variant of Chlamydia trachomatis )
- Chlamydia suis Everett et al. 1999 (previously a variant of Chlamydia trachomatis )
- Chlamydia trachomatis ( Busacca 1935) Rake 1957 emend. Everett et al. 1999.
-
Chlamydophila Everett et al. 1999, gen. Nov.
- Chlamydophila caviae Everett et al. 1999, sp. nov. (previously a variant of Chlamydia psittaci )
- Chlamydophila pecorum ( Fukushi & Hirai 1992) Everett et al. 1999, comb. nov. (previously Chlamydia pecorum )
- Chlamydophila pneumoniae ( Grayston et al. 1989) Everett et al. 1999, comb. nov. (previously Chlamydia pneumoniae )
- Chlamydophila psittaci ( Lillie 1930) Everett et al. 1999, comb. nov. (previously Chlamydia psittaci )
Medical importance
Overview of pathogenic species
All known species of the family are pathogens in humans and animals. An infection with chlamydia is called chlamydiosis , depending on the type or even serotype involved, there are different diseases, only an overview is to be given here.
| Species or serovars | Diseases or information |
|---|---|
| Chlamydia trachomatis | C. trachomatis has several subgroups, which differ in their surface structure and are thus divided into serovars (serotypes) . These serovars, in turn, can be divided into three groups that cause different diseases. |
| C. trachomatis serovars A to C |
Serovars A to C cause trachoma , a serious form of conjunctivitis ( conjunctivitis ). It is one of the most common eye diseases in developing countries, where it often leads to blindness. In Europe, however, trachoma is rare. The transmission occurs mainly through poor hygiene. |
| C. trachomatis serovars D to K |
Serovars D to K are the most common subgroup in industrialized countries and can trigger infections in the genital area, which are often simply referred to as chlamydiosis . It is a sexually transmitted disease . Chlamydial conjunctivitis and pneumonia in newborns and paratrachoma ( swimming pool conjunctivitis ) in adults are also caused by these serovars. In rare cases, men can develop reactive arthritis (in connection with Reiter's disease ) as a long-term consequence of an infection . |
| C. trachomatis serovars L1 to L3 |
Serovars L1 to L3 trigger the lymphogranuloma venereum , which can be the cause of ulcerative proctitis, as well as painful swelling of the lymph nodes in the groin, urethritis or epididymitis. |
| Chlamydophila pneumoniae | C. pneumoniae , which is much more widespread than C. trachomatis , mainly causes inflammation of the lungs ( pneumonia ). It can also trigger chronic coughs, among other things, and is also suspected of playing a role in heart disease due to arteriosclerosis . |
| Chlamydophila psittaci |
C. psittaci is the causative agent of ornithosis (parrot disease ). The natural reservoir of C. psittaci are birds, especially parrots , which can themselves be affected by the infection. As a zoonosis , it can be transferred to humans. The chain of infection usually ends there. Birds, including from animal farms, but also parrots and pigeons play the most important role as sources of infection for humans. This condition is very rare in humans and is similar to severe pneumonia. |
| Chlamydia felis ( Chlamydophila felis ) | In addition to Bordetella bronchiseptica , C. felis is a bacterial pathogen causing cat flu . The infectious disease caused by C. felis is also known as feline chlamydiosis , it is also transmitted to humans in individual cases. |
| Chlamydophila caviae | C. caviae is typical of members of the genus Cavia (real guinea pigs), which also includes house guinea pigs . It can be found in the conjunctiva of the eye and causes conjunctivitis . |
| Chlamydophila pecorum | C. pecorum causes diseases of the urogenital tract (urinary and genital organs) in koalas , which can lead to infertility. In other animal species it has been associated with miscarriages ( abortions ), conjunctivitis , encephalomyelitis , enteritis , pneumonia, and polyarthritis . |
| Chlamydia abortus ( Chlamydophila abortus ) | C. abortus caused in sheep and goats the enzootic abortion . Here, too, it may be a zoonosis associated with a severe fever; in individual cases, the bacterium was detected in women who work with sheep, specifically infected lambing ewes, and who suffered a miscarriage. |
pregnancy
| Classification according to ICD-10 | |
|---|---|
| O98.3 | Other infections, mainly transmitted through sexual intercourse, that complicate pregnancy, childbirth and the puerperium |
| A56.- | Other sexually transmitted chlamydial diseases |
| P23.1 | Congenital pneumonia due to chlamydia |
| P39.1 | Chlamydial conjunctivitis in the newborn |
| ICD-10 online (WHO version 2019) | |
Chlamydia is usually transmitted from mother to child during the birth process. Transmission in the uterus is possible, but rare. Infection can lead to severe pneumonia and eye infections in the newborn child , which can lead to blindness . If left untreated, the risk of miscarriage , stillbirth or premature birth increases .
therapy
Chlamydial infections are usually treated with tetracyclines (e.g. doxycycline ) or with macrolides (e.g. erythromycin or azithromycin ) in pregnant women and children. β-lactam antibiotics such as penicillin are completely ineffective due to the lack of a cell wall, as they require a cell wall for their mechanism of action. Antibiotic resistance is rather rare in chlamydia, which is why the effective therapy with quinolone antibiotics , which is sometimes associated with undesirable drug effects, is often refrained from. Further information on the treatments can be found under the diseases mentioned. There is no vaccination against chlamydia. Several studies are currently ongoing.
swell
literature
- Karin D. Everett, Robin M. Bush, Arthur A. Andersen: Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. In: International journal of systematic bacteriology. Volume 49, No. 2, April 1999, pp. 415-440, ISSN 0020-7713 . PMID 10319462 .
- Michael T. Madigan, John M. Martinko, Jack Parker: Brock Microbiology. German translation edited by Werner Goebel, 1st edition. Spektrum Akademischer Verlag GmbH, Heidelberg / Berlin 2000, ISBN 978-3-8274-0566-1 .
- Helmut Hahn, Stefan HE Kaufmann, Thomas F. Schulz, Sebastian Suerbaum (eds.): Medical microbiology and infectiology . 6th edition. Springer Verlag, Heidelberg 2009, ISBN 978-3-540-46359-7 .
- Marianne Abele-Horn: Antimicrobial Therapy. Decision support for the treatment and prophylaxis of infectious diseases. With the collaboration of Werner Heinz, Hartwig Klinker, Johann Schurz and August Stich, 2nd, revised and expanded edition. Peter Wiehl, Marburg 2009, ISBN 978-3-927219-14-4 , pp. 189-191.
Individual evidence
- ↑ a b c d e f g Michael T. Madigan, John M. Martinko, Jack Parker: Brock Mikrobiologie. German translation edited by Werner Goebel, 1st edition. Spectrum Akademischer Verlag GmbH, Heidelberg / Berlin 2000, ISBN 978-3-8274-0566-1 , pp. 588-591.
- ↑ a b c d e f g h i Herbert Hof, Rüdiger Dörries: Dual series: Medical microbiology . 3. Edition. Thieme Verlag, Stuttgart 2005, ISBN 978-3-13-125313-2 , p. 447-451 .
- ↑ a b c d e f g h Helmut Hahn, Stefan HE Kaufmann, Thomas F. Schulz, Sebastian Suerbaum (eds.): Medical microbiology and infectiology . 6th edition. Springer Verlag, Heidelberg 2009, ISBN 978-3-540-46359-7 , p. 416-426 .
- ↑ a b c d e f g h i j k l m n o p q r s KD Everett, RM Bush, AA Andersen: Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. In: International journal of systematic bacteriology. Volume 49, No. 2, April 1999, pp. 415-440, ISSN 0020-7713 . PMID 10319462 .
- ↑ David M. Ojcius, Toni Darville, Patrik M. Bavoil: The secret plague . In: Spectrum of Science . No. 2, February 2006. Spektrum der Wissenschaft Verlag, pp. 28-35, ISSN 0170-2971 .
- ↑ a b H. Okuda, K. Ohya et al. a .: Detection of Chlamydophila psittaci by using SYBR green real-time PCR. In: The Journal of veterinary medical science / the Japanese Society of Veterinary Science. Volume 73, No. 2, February 2011, pp. 249-254, ISSN 0916-7250 . PMID 20948172 .
- ↑ TRBA (Technical Rules for Biological Agents) 466: Classification of prokaryotes (Bacteria and Archaea) into risk groups. In: Website of the Federal Institute for Occupational Safety and Health (BAuA). April 25, 2012, p. 54 , accessed March 9, 2013 .
- ↑ a b c d e f g Chlamydioses (Part 2): Diseases caused by Chlamydophila psittaci, Chlamydophila pneumoniae and Simkania negevensis - RKI-Ratgeber. (No longer available online.) In: Website of the Robert Koch Institute (RKI). March 8, 2010, archived from the original on July 16, 2015 ; accessed on May 31, 2018 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ a b c d e Chlamydioses (Part 1): Diseases caused by Chlamydia trachomatis - RKI advice. In: Website of the Robert Koch Institute (RKI). December 21, 2010, accessed May 31, 2018 .
- ↑ Prevalence and incidence of selected sexually transmitted infections. In: website of the World Health Organization (WHO) . WHO, Department of Reproductive Health and Research, 2011, accessed November 23, 2013 .
- ↑ a b S. Van Lent, JR Piet u. a .: Full genome sequences of all nine Chlamydia psittaci genotype reference strains. In: Journal of bacteriology. Volume 194, No. 24, December 2012, pp. 6930-6931, ISSN 1098-5530 . doi : 10.1128 / JB.01828-12 . PMID 23209198 . PMC 3510619 (free full text).
- ↑ a b c d Jean Euzéby, Aidan C. Parte: Family Chlamydiaceae. In: List of Prokaryotic names with Standing in Nomenclature ( LPSN ). Accessed May 31, 2018 .
- ↑ RS Gupta, V. Bhandari, HS Naushad: Molecular Signatures for the PVC Clade (Planctomycetes, Verrucomicrobia, Chlamydiae, and Lentisphaerae) of Bacteria Provide Insights into Their Evolutionary Relationships. In: Frontiers in microbiology. Volume 3, 2012, p. 327, ISSN 1664-302X . doi : 10.3389 / fmicb.2012.00327 . PMID 23060863 . PMC 3444138 (free full text).
- ↑ a b J. Schachter, RS Stephens u. a .: Radical changes to chlamydial taxonomy are not necessary just yet. In: International journal of systematic and evolutionary microbiology. Volume 51, No. 1, January 2001, p. 249; author reply pp. 251-253 , ISSN 1466-5026 . doi : 10.1099 / 00207713-51-1-249 , PMID 11211265 .
- ^ RS Stephens, G. Myers u. a .: Divergence without difference: phylogenetics and taxonomy of Chlamydia resolved. In: FEMS immunology and medical microbiology. Vol. 55, No. 2, March 2009, pp. 115-119, ISSN 1574-695X . doi : 10.1111 / j.1574-695X.2008.00516.x . PMID 19281563 . (Review).
- ↑ a b Konrad Sachse, Patrik M. Bavoil u. a .: Emendation of the family Chlamydiaceae: Proposal of a single genus, Chlamydia, to include all currently recognized species. In: Systematic and Applied Microbiology. Volume 38, No. 2, March 2015, pp. 99-103, doi : 10.1016 / j.syapm.2014.12.004 .
- ^ G. Greub: International Committee on Systematics of Prokaryotes. Subcommittee on the taxonomy of the Chlamydiae: Minutes of the inaugural closed meeting, 21 March 2009, Little Rock, AR, USA. In: International journal of systematic and evolutionary microbiology. Volume 60, No. 11, November 2010, pp. 2691-2693, ISSN 1466-5034 . doi : 10.1099 / ijs.0.028225-0 . PMID 21048221 .
- ^ G. Greub: International Committee on Systematics of Prokaryotes. Subcommittee on the taxonomy of the Chlamydiae: Minutes of the closed meeting, June 21, 2010, Hof bei Salzburg, Austria. In: International journal of systematic and evolutionary microbiology. Volume 60, No. 11, November 2010, p. 2694, ISSN 1466-5034 . doi : 10.1099 / ijs.0.028233-0 . PMID 21048222 .
- ^ G. Greub: International Committee on Systematics of Prokaryotes. Subcommittee on the taxonomy of Chlamydiae: Minutes of the closed meeting, 23 February 2011, Ascona, Switzerland. In: International journal of systematic and evolutionary microbiology. Volume 63, No. 5, May 2013, pp. 1934-1935, ISSN 1466-5026 . doi : 10.1099 / ijs.0.028225-0 .
- ↑ Matthias Horn, Michael Wagner: Bacteria that live in Acanthamoeba: Dasein in Hidden. In: Biology in Our Time. Volume 31, No. 3, May 2001, pp. 160-168, doi : 10.1002 / 1521-415X (200105) 31: 3 <160 :: AID-BIUZ160> 3.0.CO; 2-T .
- ↑ Marianne Abele-Horn (2009), p. 191.
- ↑ Chlamydia in pregnancy. In: HealthExpress website. January 31, 2017, accessed February 26, 2017 .
- ↑ Two vaccines against chlamydia successfully tested in mice. In: Deutsches Ärzteblatt . Deutscher Ärzteverlag GmbH, editorial office of Deutsches Ärzteblatt, July 26, 2016, accessed on May 31, 2018 .
Web links
- Portal with scientific information on chlamydia (English). (No longer available online.) December 9, 2011, archived from the original on June 27, 2015 ; Retrieved November 26, 2013 .
- Chlamydiae.com: The comprehensive reference and education wiki on Chlamydia and the Chlamydiales .