Bordetella bronchiseptica
| Bordetella bronchiseptica | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|

Bordetella bronchiseptica |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name | ||||||||||||
| Bordetella bronchiseptica | ||||||||||||
| ( Ferry 1912) Moreno-Lopez 1952 |
Bordetella bronchiseptica is a bacterium from the genus Bordetella , which is ofgreat veterinary importanceas a causative agent of various animal diseases. They are small, gram-negative rods that are difficult to distinguish from the related species Bordetella pertussis and Bordetella parapertussis (the pathogens that cause whooping cough ). The cells grow strictly aerobically , so they need oxygen to multiply. When culturing on nutrient media , a change in the appearance of the colonies is sometimesobserved, which is accompanied by a change in the virulent behavior of Bordetella bronchiseptica .
When Bordetella bronchiseptica was discovered , it was wrongly assumed that it is the pathogen causing distemper in dogs . It was only later recognized that they caused a bacterial infectious disease of the respiratory tract in several mammals (e.g. dogs, cats and pigs ) . Often other bacteria or viruses are also involved in these diseases . From a transmission to humans has been reported in individual cases, however, this mainly affects patients with a weakened immune system . For prevention in domestic and farm animals a vaccine possible.
Bordetella bronchiseptica was discovered by Newell S. Ferry in 1911 and initially referred to as Bacillus bronchicanis . The bacterium was also named Bacillus bronchisepticus and is also known by other synonyms . The genome of the Bordetella bronchiseptica RB50 bacterial strain was completely sequenced in 2003 .
features
Appearance

The cells of Bordetella bronchiseptica are short to cocoid rods . They are gram negative . In contrast to Bordetella pertussis , the species is motile , it can move independently with the help of peritrichally arranged flagella . Endospores as persistence forms are not formed. The cells have pili (fimbriae) on their surface and are surrounded by a capsule . Like other representatives of the Bordetella genus, they appear individually, in pairs or in groups in the light microscope image and are difficult to distinguish from Haemophilus species.
On solid nutrient media , the cells grow into very small, transparent colonies with a diameter of 0.5–1.0 mm. Compared to Bordetella parapertussis , the colonies are even smaller. On blood agar a place hemolysis place, this also applies to the related species B. pertussis and B. parapertussis . A pigment formation on other growth media does not occur. The colonies are subject to the four-phase SR modulation, whereby the change from the S to the R form can occur spontaneously. The abbreviations S and R stand for the appearance of the colonies, from English smooth , "smooth" and English rough , "rough" (see Griffith's experiment ).
- Phase I → The colonies grow in the S-shape, the cells form a capsule, are haemolytic and virulent .
- Phase II → transitional form
- Phase III → transitional form, the cells do not form a capsule.
- Phase IV → The colonies develop the R-shape, the cells are not surrounded by a capsule, they are not virulent.
Growth and metabolism
The metabolism of Bordetella bronchiseptica is based on breathing , the species is strictly aerobic , so it needs oxygen to grow. The catalase test and the oxidase test are positive. Furthermore, the metabolism is to be characterized as chemoorganotrophic and heterotrophic , B. bronchiseptica uses organic compounds as an energy source and also to build up cellular substances. It is asaccharolytic, i.e. H. it cannot utilize sugar (e.g. glucose ); instead, amino acids are among the substrates that are broken down. This must be taken into account when choosing the suitable culture medium for cultivation, whereby B. bronchiseptica is not as demanding as B. pertussis .
The optimal temperature for growth is 35–37 ° C. Growth takes place in a temperature range of 15–37 ° C. Growth is variable at temperatures below (10 ° C) or above (44 ° C), which means that some of the bacterial strains examined are still able to multiply. At 15 ° C it takes about 10 days for colonies to be seen, at 35 ° C it is usually 1–2 days to incubate . B. bronchiseptica can tolerate small amounts of sodium chloride (table salt) in the nutrient medium. Growth is possible with a content of 6% sodium chloride, only at a content of 7.5% NaCl or more no growth occurs. It is not halophilic as it can reproduce in the absence of sodium chloride. Growth also occurs in the presence of bile salts , a content of 10% is tolerated, and even with a content of 40% bile salts in the nutrient medium, growth still takes place in some of the strains examined.
Biochemical features, such as the enzymes present and the resulting metabolic properties, can be used in a colorful series to identify B. bronchiseptica . In addition to the positive catalase and oxidase test, the following features can be used: It behaves positively in the test for nitrate reduction , it can consequently reduce nitrate to nitrite . The urease test is positive for many strains, so they have the enzyme urease and are able to break down urea . However, there are several urease-negative strains, so the test result for the species is reported as variable. Gelatin , casein or starch cannot be broken down by hydrolysis . Nor is it capable of aesculin hydrolysis . It has the enzyme arginine dihydrolase (ADH) and can therefore break down the amino acid arginine , and tyrosine can also be hydrolyzed. It can also break down the amino acids L - glutamic acid and L - proline .
Further organic compounds that can be used as a source of energy and to build up the cells' own substances are citrate , pyruvate , succinate , acetate , adipate and itaconate . Hydrogen sulfide (H 2 S) is not formed. The Voges-Proskauer test for acetoin formation and the indole test are negative. Since no carbohydrates are broken down, there is no acid formation either, so the methyl red test is also negative. The differentiation from B. parapertussis and B. pertussis is difficult because the three species show similarities in many metabolic-physiological and biochemical characteristics, but they can be differentiated on the basis of some characteristics (see overview ).
Serological characteristics
Bordetella bronchiseptica has lipopolysaccharides (LPS) deposited on its cell wall . They are part of the outer membrane and typical of gram-negative bacteria. The LPS consist of fat-like components, combined with oligosaccharides (sugar components), which act as antigen and can be used serologically for the detection, as they differ from the LPS of the related species. Proteins are also part of the outer membrane; they are often abbreviated as OMP, after the English term outer membrane proteins . They also act as an antigen and cause agglutination when they meet the appropriate antibodies .
The SR modulation already described also has an impact on the serological behavior, since some structures that act as antigens are no longer present in the R form. The virulence factors are also no longer formed by the R-form, so it is no longer virulent. A similar effect can be brought about by changing the environmental conditions. For example, when growing at low temperatures, a form with changed antigens predominates, which is also no longer virulent. Both the SR modulation and the antigen modulation are reversible and are controlled by a gene locus . The virulent strains are also referred to as Bvg + , the avirulent as Bvg - .
genetics
The genome of the bacterial strain Bordetella bronchiseptica RB50 (also listed under the name ATCC BAA-588) was completely sequenced in 2003 . This is a strain that was isolated from a rabbit as an animal pathogen . The genome has a size of 5339 kilobase pairs (kb), which corresponds to about 115% of the genome size of Escherichia coli . It exists as a circular bacterial chromosome . 4994 proteins are annotated . The comparison with the genome size of B. parapertussis (4774 kb) and B. pertussis (4086 kb) shows that the genome of B. bronchiseptica is larger. This is explained by the fact that the other two species developed from an earlier form of B. bronchiseptica and their restriction to one host (humans) has led to genes that are no longer "needed" being lost.
By 2013, the genome of five other strains - B. bronchiseptica 253, 1289, MO149, Bbr77 and D445 - had been sequenced and published. These strains have been isolated from humans or mammals . The genome sizes are slightly smaller than in the strain examined first and lie in a range from 4970 to 5264 kb. This study also sequenced strains of the related species and confirmed that their genome size is smaller. The results of the sequencing show a high GC content (the proportion of the nucleobases guanine and cytosine ) in the bacterial DNA , which is around 68 mol percent. According to the results of the genome studies in 2013, no B. bronchiseptica strain contains a plasmid ; this peculiarity was only found in B. parapertussis Bpp5 ( isolated from a sheep ).
A study from 1997 came to a different conclusion. According to this, 52 antibiotic-resistant strains isolated from cats were examined and plasmids were found in ten. With the help of conjugation experiments an attempt was made to transfer the plasmids into the strain Escherichia coli K12, which was only successful with two plasmids. These plasmids are each 51 kb in size and carry the genetic information for antibiotic resistance to ampicillin , tetracycline , streptomycin and sulfonamides . The resistance is based on the one hand on the formation of a penicillinase that cleaves the β- lactam ring of ampicillin and, in the case of tetracycline resistance, on an efflux mechanism .
The genes for important enzymes involved in sugar metabolism are missing in the genome of the three “classic” species. This applies to glucokinase and phosphofructokinase (enzymes of glycolysis ), as well as fructose-1,6-bisphosphatase , which is involved in the pentose phosphate pathway , among other things . This explains the asaccharolytic metabolism of B. bronchiseptica . The enzyme urease is also the subject of genetic studies. In B. bronchiseptica many strains are urease-positive, so they have the enzyme, but also urease-negative strains occur. The urease operon consists of structural genes and additional genes, two of which (ureE and ureF) are fused to one another, thereby forming a fusion protein (UreEF). Furthermore, a regulator gene ( B. bronchiseptica urease regulator, BbuR) can be found, which controls the gene expression of the urease operon and is associated with the protection of the bacterium from lysosomal damage. In the promoter of BbuR occurring mutations therefore are responsible for the different behavior of the expression of urease.
proof
For cultivation simple culture media are suitable if it amino acids or peptone included. Blood agar is often used for cultivation, as it is for other Bordetelles . However, Bordetella bronchiseptica shows no haemolysis if horse blood is used; if sheep blood is used, haemolysis only occurs in colonies of phase I. The culture media Bordet Gengou blood agar and Regan Lowe suturing medium used for B. parapertussis are also suitable. Similarly, MacConkey agar and Salmonella-Shigella agar be used. Colonies can be seen after incubation for 1-2 days at 35-37 ° C. The bacterial culture grown on the nutrient media can then be examined biochemically in order to distinguish it from the related Bordetella species.
It has been discussed in the past whether only urease-positive strains can be considered virulent. This could be refuted by animal experiments with mice and guinea pigs as model organisms . It was shown that the expression of urease has no influence on the settlement and retention of the bacterium in the respiratory tract of mice. A urease-negative mutant of B. bronchiseptica was also able to colonize the respiratory and digestive tracts of guinea pigs. Nevertheless, an investigation of the phenotypic characteristics of urease activity and the affinity for Congo red is recommended in order to identify virulent B. bronchiseptica strains (Bvg + ). The formation of virulence factors was compared with this and it was found that a virulent phenotype was found with a binding of Congo red of at least 26 n mol / ml ( nanomoles per milliliter) and a urease activity of less than 2.6 U (unit for specifying the enzyme activity) present.
In addition to serological evidence, molecular biological methods are also used. With the help of the PCR process ( polymerase chain reaction ) certain parts of the bacterial genome are detected, which is much more specific than serological or biochemical test methods. A method developed in France in 2013 is based on Real Time Quantitative PCR ( q-PCR ) and aims to detect B. bronchiseptica and B. parapertussis , which can thus be detected and distinguished from one another.
Occurrence
The habitat of Bordetella bronchiseptica is the respiratory tract of various mammals, including dogs , cats , pigs , horses , rabbits , hamsters , rats , mice and guinea pigs, where it can cause respiratory problems. It can also be found in humans, but this is rare and is attributed to close contact with a sick animal. Unlike the related species, B. bronchiseptica can survive in the environment for a longer period of time.
discovery
Bordetella bronchiseptica was named Bacillus bronchicanis in a 1911 paper by Newell S. Ferry . Ferry was on the lookout for distemper, a pathogen that caused distemper, and studied dogs that had it for several years. At the time, symptoms of distemper's respiratory disease were compared to whooping cough . Ferry took swabs of the eye and nasal secretions and was able to detect several different types of bacteria after cultivation, including staphylococci , streptococci and bacilli . He classified this as a causative agent of a secondary infection and hypothesized that many deaths were actually due to these bacteria and not to the actual pathogen, distemper. Through the autopsy of killed dogs and the microbiological examination of samples from lung, trachea and larynx tissue, Newell S. Ferry succeeded for the first time in 1908 in growing pure cultures of a "Bacillus". In his report he mentioned that the colonies grow very slowly and after 24 hours they can only be seen on the nutrient medium with the help of a magnifying glass. He called this bacterium Bacillus bronchicanis and described the appearance of the cells in the studies at that time.
To prove that the isolated bacterium actually caused the disease, Ferry followed Koch's postulates . To this end, he carried out numerous animal experiments with young dogs. He made sure that the dogs' surroundings were as sterile as possible . They were housed in cleaned laboratory rooms, to which only a limited number of people had access, to which disinfection measures were also intended to ensure that they did not carry other pathogens as contaminants . The dogs judged to be healthy were then infected with the bacterium in a liquid nutrient medium, for example via the nostrils, and reports were made of any diseases that had occurred. An attempt was then made to isolate Bacillus bronchicanis from the dead or killed animals . This was successful in many cases, but with the restriction that the samples came from animals at an early stage of the disease that did develop respiratory diseases but did not yet suffer from discharge from the eyes or nose. Newell S. Ferry was of the opinion that his investigations conclusively proved that Bacillus bronchicanis is the causative agent of distemper. In fact, it is a virus, the canine distemper virus , which was discovered by Henri Carré in 1905 . However, this was not recognized until 1926 by the studies of George William Dunkin and Patrick Laidlaw . According to his own statements, Ferry did not succeed in his investigations to detect the “filterable virus” described by Carré.
In further investigations, Ferry infected other animal species with the discovered Bacillus bronchicanis . Since these animals also developed symptoms of illness that were reminiscent of distemper, he changed the name of the pathogen to Bacillus bronchisepticus in 1912 to make it clear that the dog is not the only host. The pathogenicity of the bacterium was discovered in 1912 by other scientists in experimental animals such as cats, rabbits and guinea pigs, and the disease was initially also interpreted as a variant of the distemper that occurs in dogs. In the 1960s and 1970s, Bordetella bronchiseptica was once again detected as a pathogen in experimental animals (cats and monkeys) and described as the cause of an independent infectious disease.
Systematics
External system
Bordetella bronchiseptica is one of several species from the genus Bordetella in the family of the Alcaligenaceae , this is placed in the order of the Burkholderiales in the class of the Betaproteobacteria . The genus Alcaligenes , to which B. bronchiseptica was previously assigned, also belongs to the family Alcaligenaceae. The genus Haemophilus , which has morphological similarity to the Bordetellen, belongs to the class of the Gammaproteobacteria , while the genus Brucella , to which B. bronchiseptica was also previously assigned, belongs to the class of the Alphaproteobacteria .
Internal system
From the genus Bordetella , the species B. bronchiseptica , B. parapertussis and B. pertussis have been known since the first half of the 20th century; they are also known as "classic" Bordetella species. Other species have been newly discovered since 1984, e.g. B. B. avium . The species discovered first are strikingly similar, so that classification as subspecies is also being discussed. A comprehensive genetic study of seven bacterial strains in 2012 brought new insights into the phylogenetic relationships. Only about 50% of the "core genome" ( English pan-genome ) occurs in all strains, this diversity in the genome is considered to be the cause of different hosts or different pathogenicity factors .
After examining sick dogs and isolating a bacterium, Newell S. Ferry named it in 1911 as Bacillus bronchicanis . Shortly afterwards, he realized that the pathogen also occurs in other hosts and in 1912 changed the name to Bacillus bronchisepticus . This is considered the initial description as other scientists later referred to this name in most cases. In 1952 the genus Bordetella was established by Manuel Moreno López , to which the bacterium was then placed. B. bronchiseptica is known by numerous synonyms which are based on the fact that the bacterium was initially assigned to these genera because of its similarity to representatives of other genera (such as Alcaligenes or Bacillus ). Synonyms are Bacillus bronchicanis Ferry 1911 Bacillus bronchisepticus Ferry 1912 Bacterium bronchisepticus (Ferry 1912) Evans 1918 Alcaligenes bronchisepticus (Ferry 1912) Bergey inter alia 1925 Brucella bronchiseptica (Ferry 1912) Topley & Wilson 1929 Alcaligenes bronchicanis (Ferry 1911) main 1935 and Haemophilus bronchisepticus (Ferry 1912) Wilson & Miles 1946.
More than 70 bacterial strains are known of the species B. bronchiseptica . The strain B. bronchiseptica ATCC 19395 is the type strain of the species. Several bacterial strains are deposited in various collections of microorganisms . So far (as of 2014) six bacterial strains have been genetically investigated, further strains are currently being researched in further genome projects.
etymology
The generic name was chosen in honor of the Belgian microbiologist Jules Bordet . The specific epithet is made up of the Latin words bronchia (" bronchia ", "airways in the lungs") and septicus , whereby septicus is derived from the Greek word sepsis ("putrefaction"). This indicates the pathogenicity of the bacterium, as it can lead to an infection of the bronchi. The name originally chosen by Ferry as Bacillus bronchicanis contains the Latin word canis for "dog", because he isolated the bacterium from this.
Human medical importance
Bordetella bronchiseptica can cause respiratory diseases in humans. Involvement as a causative agent of whooping cough is also discussed. However, it is not often found in human isolates. An infection of humans occurs rarely and is attributed to close contact with a sick animal. If an infection occurs, it particularly affects patients with weakened immune systems .
In 1999, for example, a study of HIV- infected people showed that B. bronchiseptica was detectable in bacterial cultures in nine of them . The respiratory tract was infected in eight cases , with symptoms ranging from mildly progressing diseases of the upper respiratory tract to pneumonia . Two patients had contact with dogs in their household, one patient with cats. A case documented in 2010 describes a patient with a kidney-pancreas transplant . As a result of the transplant, she had to take immunosuppressive drugs. After another operation, the patient developed pneumonia that could not be contained with the administered antibiotics . This was followed by a bronchoscopy , in which samples were taken in which B. bronchiseptica was detected in culture . Further investigation revealed that the dog patient recently with a live vaccine , the B. bronchiseptica has been vaccinated. However, there was no comparison of the genetic material from the vaccine strain and the strain found in the patient, so there is no evidence that she was infected by the vaccine.
Patients with cystic fibrosis (cystic fibrosis) can B.bronchiseptica be at risk. A report published in 2014 describes the cases of seven children with cystic fibrosis in whom B. bronchiseptica was detected 23 times . The bacterial infection made the symptoms of the disease worse. As a consequence of the cases described above, reference is made to the possible transmission of the pathogen from pets to humans, which is assessed as a risk for immunosuppressed patients.
The Biological Agents Ordinance comes to a similar assessment : B. bronchiseptica is pathogenic (“pathogenic”) to humans ; it is assigned to risk group 2 by the Biological Agents Ordinance in conjunction with the TRBA ( Technical Rules for Biological Agents) 466 . Furthermore, it is noted in the classification that it is pathogenic for humans and vertebrates, but that normally there is no transmission between the two host groups, so that it is not a zoonotic agent .
Veterinary importance
Bordetella bronchiseptica is of greater importance in veterinary medicine , as it causes infections of the respiratory tract as a pathogen in many mammals, both domestic animals and wild animals .
Pathogenicity
Similar to an infection with B. parapertussis and B. pertussis in humans, the ciliated epithelial cells of the respiratory tract are the target of the pathogen. This was researched, among other things, in cell cultures with cells isolated from dogs. After contact with virulent B. bronchiseptica (phase I form), these attach to the cilia, the frequency of which is reduced significantly within 5 minutes, and within 3 hours there is no longer any movement of the ciliated epithelium (ciliostasis). As a result of the ciliostasis, the bronchial mucus that has formed can no longer be removed from the respiratory tract.
Bordetella pertussis , better known as a pathogen in humans , has numerous virulence factors such as filamentous hemagglutinin (FHA) and pertussis toxin (PT), a protein that acts as an exotoxin and adhesin . Most of these virulence factors are also found in B. bronchiseptica , although some of this is still the subject of research. Genes were identified in the genome that code for the pertussis toxin, but these are not expressed , so the protein is not produced. The FHA is also formed by B. bronchiseptica . It works as an adhesin and binds directly to the glycosphingolipids in the cell membrane of the cilia.
Furthermore, the heat-labile toxin, the invasive find adenylate cyclase , the tracheal cytotoxin (TCT) and a so-called O-antigen and endotoxin acting lipopolysaccharides from the outer membrane in B. parapertussis , B. pertussis and B. bronchiseptica . A skin-necrotizing effect is ascribed to the heat-labile toxin . The effects of adenylate cyclase and tracheal cytotoxin are responsible for ciliostasis. In B. bronchiseptica , pertactin also plays a role as an adhesin. It is also believed that the fimbriae and proteins in the outer membrane (OMP) also contribute to binding to the host cell. This was confirmed by the genetic studies. In gram-negative pathogenic bacteria (. E.g., Vibrio parahaemolyticus ) is often found a type III secretion system (engl. Type III secretion system , as TTSS hereinafter). The TTSS is an important factor for the pathogenicity, because with its help bacterial toxins are introduced into the cells of the host in a targeted manner . Also B. bronchiseptica has a type III secretion system, whose structure and function is not yet clear.
Sources of infection
The respiratory tract is the habitat of Bordetella bronchiseptica . The path of infection is a droplet infection , the pathogen is transmitted through droplets that sick animals cough up or sneeze out. Since B. bronchiseptica can survive in the environment earlier than the related species, smear infection cannot be ruled out.
Infectious diseases
→ Main articles: Kennel cough , cat flu , rhinitis atrophicans and contagious rabbit flu
Various mammals can contract an infection by Bordetella bronchiseptica , mostly affecting the respiratory tract (respiratory tract) or parts of it. The disease manifests itself as a runny nose , acute bronchitis and pneumonia and is also known in veterinary medicine as bordetellosis ( English bordetellosis ), z. B. as Feline Bordetellose. The course of the disease can vary widely, from mild respiratory diseases to pneumonia with fatal consequences, with young animals being particularly at risk. It should be noted that the infectious diseases mentioned are all complex and are usually caused by more than one pathogen. Here is an overview of the animal groups affected and the infectious diseases caused by B. bronchiseptica :
- Infections in dogs : The disease manifests itself as tracheobronchitis - in addition to the bronchi, the windpipe (trachea) is also affected - and is known as kennel cough . In addition to B. bronchiseptica , it can also be caused by various viruses, in particular the canine parainfluenza virus type 2.
- Infections in cats : Viruses are involved in cat flu , a feline infectious disease of the upper respiratory tract, but also bacteria such as Chlamydophila felis and B. bronchiseptica . The latter was isolated from cats with respiratory diseases in breeding stations; the cats were free from the viral pathogens FeHV-1 and FCV. The infection can be through inhalation one with B. bronchiseptica offset aerosol provoke. In kittens , this leads to symptoms of respiratory illness with nasal discharge, sneezing, coughing and rattling noises during auscultation .
- Infections in pigs : it was originally assumed that B. bronchiseptica caused rhinitis atrophicans ("sniffing disease"), a disease in domestic pigs characterized by the destruction ( atrophy ) of the turbinates . Later studies, however, identified Pasteurella multocida as the pathogen, with B. bronchiseptica playing a role as a pioneer. However, it was possible to show in animal experiments that an isolated infection with B. bronchiseptica leads to atrophy of the bones in the turbinates after 28 days. Bordetellos is particularly problematic for piglets up to four weeks old. They usually become infected with the latently infected sow and develop pneumonia. This disease is independent of the actual pathogen, for MIRD complex ( English Mycoplasma induces respiratory disease , " Mycoplasma -induced airway disease" ) is counted. The infection is favored by intensive animal husbandry. Rhinitis atrophicans is a reportable animal disease .
- Infections in rabbits : Here, too, B. bronchiseptica was initially assumed to be the pathogen for the contagious rabbit cold, later results indicate an infection by Pasteurella multocida , with B. bronchiseptica once again being viewed as a pioneer for the infection. In addition to a runny nose, symptoms include otitis media and tracheobronchitis.
- Infections in guinea pigs and rats : Here the symptoms are similar to those in rabbits; in addition to B. bronchiseptica , P. multocida is a typical pathogen (see respiratory diseases in guinea pigs ). Also, abortions and stillbirths can be a consequence of infection. In experimental animal facilities, B. bronchiseptica infection spreads rapidly and leads to a high number of sick animals, many of which die.
therapy
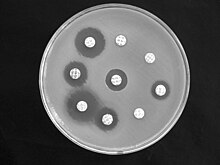
Microbiological methods, for example an antibiogram or the determination of the minimum inhibitory concentration, can be used to determine in vitro which antibiotics Bordetella bronchiseptica is resistant to and to which it reacts sensitively; these can then be used in therapy if necessary. Studies from 1977 on 50 bacterial strains showed that they are all sensitive to polymyxin B , chloramphenicol and tetracyclines . Most strains continue to react sensitively to the aminoglycoside antibiotics gentamicin and kanamycin , the majority (between 70 and 66%) to nalidixic acid (from the group of quinolone antibiotics ), cefalotin (from the group of cephalosporins ), ampicillin (a semi-synthetic aminopenicillin ) and a combination of sulfonamides with trimethoprim . All strains, however, are resistant to streptomycin and penicillin G .
In 1991 the results of several research groups on this topic were evaluated, according to which there is also a sensitivity of B. bronchiseptica for the aminoglycoside antibiotics amikacin and tobramycin , as well as the semi-synthetic penicillins azlocillin , mezlocillin , piperacillin and ticarcillin . A sensitivity for cephalosporins such as cefoperazone and ceftazidime was also determined. Penicillin G was rated as not very effective, but also ampicillin, and clindamycin and erythromycin as not at all effective .
Antibiotics are often used in veterinary medicine without the pathogen being identified. Treatment with tetracyclines, especially doxycycline, is recommended in cats with Bordetellosis . In pigs, the use of aminoglycosides, fluoroquinolones, macrolides (e.g. tylosin ), tetracyclines or the combination of trimethoprim and sulfamethoxazole (also known as cotrimoxazole ) is recommended. However, an antibiogram is helpful in determining the effectiveness of the antibiotic on the bacterium in advance. Tetracyclines, ampicillin and cotrimoxazole are generally used to treat infections of the upper respiratory tract in pets. This should be questioned critically in the case of an infection with B. bronchiseptica , as resistance to trimethoprim and ampicillin occurs in numerous bacterial strains.
prevention
A vaccination can be used as a preventive measure ; in cats, for example, a vaccine based on the fimbrial antigens of B. bronchiseptica was tested as early as 1993 . Vaccination is recommended for cats if several animals are kept in a confined space, e.g. B. in an animal shelter or a boarding house. This recommendation can also be found in the guideline on the vaccination of small animals , which is published by the Standing Vet. Vaccination Commission (Vet. As an abbreviation for veterinary medicine). Another reason for vaccination mentioned there is contact with other animal species that can also be infected by B. bronchiseptica , as is the case with dogs. A vaccine for cats has been approved in Germany since 2002. The monovalent preparation called Nobivac Bb is a live vaccine that contains the bacterial strain B. bronchiseptica B-C2 and is administered intranasally , i.e. through the nose. Studies in the USA have shown that intranasal application of a live vaccine containing modified FeHV-1 and FCV (viruses that are involved in the cat flu complex) also gives the animals immunity to B. bronchiseptica , although it is not in the vaccine is included. The cats show decreased symptoms or the disease does not break out. This is explained with a non-specific immunity that is stimulated by intranasal application.
Vaccination is also only recommended for dogs under certain circumstances. These exist when they have a lot of contact with fellow dogs, for example in groups of puppies , and also when they stay in animal boarding houses or animal shelters. Vaccination is also recommended after contact with cats or other animal species that can be infected by B. bronchiseptica . A monovalent live vaccine is available for dogs in Germany, and there is also a combination product that protects against the canine parainfluenza virus (CPiV), which is also involved in the kennel cough complex. Both vaccines are given intranasally. The monovalent preparation Bronchi-Shield is an attenuated live vaccine that contains the bacterial strain B. bronchiseptica 92B and has been approved since 2011. The combination drug Nobivac BbPi has been approved since 2000 . This is also a live vaccine, containing the bacterial strain B. bronchiseptica B-C2 and the canine parainfluenza virus type 2, strain CGF.
Vaccination in rabbit breeding is recommended for rabbits in order to reduce the infection pressure in the herd. A combination preparation is available in Germany for this purpose, which contains inactivated B. bronchiseptica and Pasteurella multocida cells as a dead vaccine . It is administered subcutaneously, i.e. injected into the subcutaneous tissue. The preparation with the name CUNIVAK PAST has been approved as a vaccine against rabbit cold since 1997.
Several vaccines against rhinitis atrophicans are also available for pigs in Germany. The preparation Porcilis AR-T DF has been approved since 2000 and contains a non-toxic derivative of the P. multocida toxin as a recombinant protein and inactivated cells of B. bronchiseptica . It is used in sows to protect their piglets from the "sniffing disease". It is injected intramuscularly , i. H. injected into a muscle. Rhiniseng , which has been approved since 2010 , is also a combination preparation ; it also contains a recombinant protein from P. multocida that is no longer toxic and inactivated cells from B. bronchiseptica . The preparation Respiporc ART + EP , which has been approved since 1994, is also a combination vaccine, in addition to the derivative of the P. multocida toxin and inactivated cells of B. bronchiseptica , inactivated cells of P. multocida are also contained.
swell
literature
- Horst Finger, Carl Heinz Wirsing von König: Bordetella (Chapter 31) . In: Samuel Baron (Ed.): Medical Microbiology . 4th edition. University of Texas Medical Branch at Galveston, Galveston (TX), USA 1996, ISBN 0-9631172-1-1 ( NCBI Bookshelf ).
- BF Woolfrey, JA Moody: Human infections associated with Bordetella bronchiseptica. In: Clinical Microbiology Reviews. Volume 4, No. 3, July 1991, pp. 243-255, doi : 10.1128 / CMR.4.3.243 (currently unavailable) . PMID 1889042 . PMC 358197 (free full text). (Review).
- Karin Duchow, Katrin Hartmann u. a .: Guideline for the vaccination of small animals . Ed .: Standing Vet. Vaccination Commission in the Federal Association of Practicing Veterinarians e. V. 2nd edition. 2013, ISBN 978-3-933711-14-4 ( PDF, 504 kB [accessed March 10, 2014]).
Individual evidence
- ↑ a b c d Herbert Hof, Rüdiger Dörries: Dual series: Medical microbiology . 3. Edition. Thieme Verlag, Stuttgart 2005, ISBN 978-3-13-125313-2 , p. 408-411 .
- ↑ a b c d e f DA Bemis, HA Greisen, MJ Appel: Bacteriological variation among Bordetella bronchiseptica isolates from dogs and other species. In: Journal of clinical microbiology. Volume 5, Number 4, April 1977, pp. 471-480. PMID 858784 . PMC 274626 (free full text).
- ↑ a b c d e f Mardjan Arvand: Bordetellen . In: Helmut Hahn, Stefan HE Kaufmann, Thomas F. Schulz, Sebastian Suerbaum (eds.): Medical microbiology and infectious diseases . 6th edition. Springer Verlag, Heidelberg 2009, ISBN 978-3-540-46359-7 , p. 302-307 .
- ↑ a b c d e f g h i j k Horst Finger, Carl Heinz Wirsing von König: Bordetella (Chapter 31) . In: Samuel Baron (Ed.): Medical Microbiology . 4th edition. University of Texas Medical Branch at Galveston, Galveston (TX), USA 1996, ISBN 0-9631172-1-1 .
- ↑ a b c d e f g h i j k l m n o p BF Woolfrey, JA Moody: Human infections associated with Bordetella bronchiseptica. In: Clinical Microbiology Reviews. Volume 4, No. 3, July 1991, pp. 243-255, doi : 10.1128 / CMR.4.3.243 (currently unavailable) . PMID 1889042 . PMC 358197 (free full text). (Review).
- ↑ a b c d e f g h i j k R. Johnson, PHA Sneath: Taxonomy of Bordetella and Related Organisms of the Families Achromobacteraceae, Brucellaceae, and Neisseriaceae. In: International Journal of Systematic Bacteriology. Volume 23, No. 4, October 1973, pp. 381-404. doi : 10.1099 / 00207713-23-4-381 .
- ↑ a b c d DA Bemis, JR Kennedy: An improved system for studying the effect of Bordetella bronchiseptica on the ciliary activity of canine tracheal epithelial cells. In: The Journal of Infectious Diseases. Volume 144, No. 4, October 1981, pp. 349-357. PMID 7288215 .
- ↑ F. von Wintzingerode, A. Schattke u. a .: Bordetella petrii sp. nov., isolated from an anaerobic bioreactor, and emended description of the genus Bordetella. In: International journal of systematic and evolutionary microbiology. Volume 51, No. 4, July 2001, pp. 1257-1265, ISSN 1466-5026 . doi : 10.1099 / 00207713-51-4-1257 . PMID 11491321 .
- ↑ a b Bordetella bronchiseptica RB50. In: Website Genomes Online Database (GOLD) . Retrieved February 28, 2014 .
- ↑ a b c K. Kersters, K.-H. Hinz u. a .: Bordetella avium sp. nov., Isolated from the Respiratory Tracts of Turkeys and Other Birds. In: International Journal of Systematic Bacteriology. Volume 34, No. 1, January 1984, pp. 56-70. doi : 10.1099 / 00207713-34-1-56 .
- ↑ a b LE Friedman, BN de Rossi u. a .: Phenotype evaluation of Bordetella bronchiseptica cultures by urease activity and Congo red affinity. In: Letters in applied microbiology. Volume 33, No. 4, October 2001, pp. 285-290, ISSN 0266-8254 . PMID 11559402 .
- ↑ a b c d e f g h J. Parkhill, M. Sebaihia u. a .: Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. In: Nature genetics. Volume 35, No. 1, September 2003, pp. 32-40, ISSN 1061-4036 . doi : 10.1038 / ng1227 . PMID 12910271 .
- ↑ a b c d e Bordetella bronchiseptica. In: National Center for Biotechnology Information (NCBI) Genome website . Retrieved March 1, 2014 .
- ↑ a b c d e J. Park, Y. Zhang u. a .: Comparative genomics of the classical Bordetella subspecies: the evolution and exchange of virulence-associated diversity amongst closely related pathogens. In: BMC genomics. Volume 13, October 2012, p. 545, ISSN 1471-2164 . doi : 10.1186 / 1471-2164-13-545 . PMID 23051057 . PMC 3533505 (free full text).
- ↑ a b AJ Speakman, SH Binns u. a .: Characterization of antibiotic resistance plasmids from Bordetella bronchiseptica. In: The Journal of antimicrobial chemotherapy. Volume 40, No. 6, December 1997, pp. 811-816, ISSN 0305-7453 . PMID 9462432 .
- ^ DJ McMillan, M. Mau, MJ Walker: Characterization of the urease gene cluster in Bordetella bronchiseptica. In: Genes. Volume 208, No. 2, February 1998, pp. 243-251, ISSN 0378-1119 . PMID 9524276 .
- ↑ DJ McMillan, E. Medina et al. a .: Expression of urease does not affect the ability of Bordetella bronchiseptica to colonize and persist in the murine respiratory tract. In: FEMS microbiology letters. Volume 178, No. 1, September 1999, pp. 7-11, ISSN 0378-1097 . PMID 10483716 .
- ↑ DM Monack, S. Falkow: Cloning of Bordetella bronchiseptica urease genes and analysis of colonization by a urease-negative mutant strain in a guinea-pig model. In: Molecular microbiology. Volume 10, No. 3, November 1993, pp. 545-553. PMID 7968532 .
- ↑ A. Tizolova, D. Brun et al. a .: Development of real-time PCR assay for differential detection of Bordetella bronchiseptica and Bordetella parapertussis. In: Diagnostic microbiology and infectious disease. [electronic publication before printing] January 2014, ISSN 1879-0070 . doi : 10.1016 / j.diagmicrobio.2013.12.020 . PMID 24525142 .
- ^ Newell S. Ferry: Etiology of Canine Distemper. In: Journal of Infectious Diseases. Volume 8, No. 4, 1911, pp. 399–420, here pp. 399–402, Internet Archive , accessed on March 14, 2014.
- ^ A b c Newell S. Ferry (1911): Etiology of Canine Distemper. Pp. 399-420, here pp. 402-405.
- ^ Newell S. Ferry (1911): Etiology of Canine Distemper. Pp. 399-420, here pp. 405-415.
- ^ Newell S. Ferry (1911): Etiology of Canine Distemper. Pp. 399-420, here pp. 418-419.
- ↑ Antje Grünberg: The canine distemper - a contribution to the history of domestic animal diseases , dissertation to obtain the degree of doctor of veterinary medicine at the Free University of Berlin . In: Clinic for cloven-hoofed animals of the veterinary medicine department of the Free University of Berlin (Ed.): Journal No. 2119 . 1997, p. 49–50 ( dissertation server FU Berlin, PDF, 284 kB [accessed on March 15, 2014]).
- ^ Newell S. Ferry: Bacillus bronchisepticus (bronchicanis): the cause of distemper in dogs and a similar disease in other animals. In: British Veterinary Journal. Volume 68, 1912, pp. 376-391.
- ^ A b c Jean Euzéby, Aidan C. Parte: Genus Bordetella. In: List of Prokaryotic names with Standing in Nomenclature Systematics of Bacteria (LPSN) . Retrieved February 28, 2014 .
- ↑ a b Jean Euzéby, Aidan C. Parte: Phylum "Proteobacteria". In: List of Prokaryotic names with Standing in Nomenclature Systematics of Bacteria (LPSN) . Retrieved February 28, 2014 .
- ↑ M. Moreno-López: El genero Bordetella [The genus Bordetella]. In: Microbiologia Española. Volume 5, 1952, pp. 177-181.
- ↑ a b Taxonomy Browser Bordetella bronchiseptica. In: National Center for Biotechnology Information (NCBI) website . Retrieved February 28, 2014 .
- ↑ Strain Passport Bordetella bronchiseptica. In: StrainInfo website (information collected about bacterial strains in over 60 biological resource centers (BRCs)). Retrieved February 28, 2014 .
- ↑ a b MS Dworkin, PS Sullivan et al. a .: Bordetella bronchiseptica infection in human immunodeficiency virus-infected patients. In: Clinical Infectious Diseases . Volume 28, No. 5, May 1999, pp. 1095-1099, ISSN 1058-4838 . doi : 10.1086 / 514761 . PMID 10452641 .
- ↑ a b JJ Gisel, LM Brumble, MM Johnson: Bordetella bronchiseptica pneumonia in a kidney-pancreas transplant patient after exposure to recently vaccinated dogs. In: Transplant infectious disease: an official journal of the Transplantation Society. Volume 12, No. 1, February 2010, pp. 73-76, ISSN 1399-3062 . doi : 10.1111 / j.1399-3062.2009.00451.x . PMID 19874567 .
- ↑ C. Brady, P. Ackerman, et al. a .: Bordetella bronchiseptica in a pediatric cystic fibrosis center. In: Journal of cystic fibrosis: official journal of the European Cystic Fibrosis Society. Volume 13, No. 1, January 2014, pp. 43-48, ISSN 1873-5010 . doi : 10.1016 / j.jcf.2013.08.002 . PMID 24011471 .
- ↑ TRBA (Technical Rules for Biological Agents) 466: Classification of prokaryotes (Bacteria and Archaea) into risk groups. In: Website of the Federal Institute for Occupational Safety and Health (BAuA) . April 25, 2012, p. 42 , accessed January 7, 2014 .
- ↑ a b c H. Egberink, D. Addie u. a .: Bordetella bronchiseptica infection in cats. ABCD guidelines on prevention and management. In: Journal of feline medicine and surgery. Volume 11, No. 7, July 2009, pp. 610-614, ISSN 1098-612X . doi : 10.1016 / j.jfms.2009.05.010 . PMID 19481041 . (Review).
- ↑ a b c d Hans-Joachim Selbitz, Uwe Truyen, Peter Valentin-Weigand: Veterinary microbiology, infection and disease theory . 8th edition. Enke Verlag, Stuttgart 2010, ISBN 978-3-8304-1080-5 , p. 169-171 .
- ↑ a b c d e Karin Duchow, Katrin Hartmann u. a .: Guideline for the vaccination of small animals . Ed .: Standing Vet. Vaccination Commission in the Federal Association of Practicing Veterinarians e. V. 2nd edition. 2013, ISBN 978-3-933711-14-4 ( download from the veterinary association ).
- ^ H. Elliott: Bordetella bronchiseptica in a closed cat colony. In: The Veterinary Record. Vol. 129, No. 21, November 1991, pp. 474-475. PMID 1763473 .
- ↑ a b AA Jacobs, WS Chalmers u. a .: Feline Bordetellosis: Challenge and Vaccine Studies. In: The Veterinary Record. Volume 133, No. 11, September 1993, pp. 260-263, ISSN 0042-4900 . PMID 8236648 .
- ↑ LA Collings, JM Rutter: Virulence of Bordetella bronchiseptica in the porcine respiratory tract. In: Journal of medical microbiology. Volume 19, No. 2, April 1985, pp. 247-255. PMID 2858594 .
- ↑ Cat vaccines. In: Website Paul Ehrlich Institute . February 13, 2014, accessed March 10, 2014 .
- ^ Nobivac Bb - live Bordetella bronchiseptica bacteria strain B-C2. In: European Medicines Agency website . October 3, 2012, accessed March 10, 2014 .
- ↑ A. Bradley, J. Kinyon et al. a .: Efficacy of intranasal administration of a modified live feline herpesvirus 1 and feline calicivirus vaccine against disease caused by Bordetella bronchiseptica after experimental challenge. In: Journal of veterinary internal medicine / American College of Veterinary Internal Medicine. Volume 26, No. 5, Sep./Oct. 2012, pp. 1121-1125, ISSN 1939-1676 . doi : 10.1111 / j.1939-1676.2012.00982.x . PMID 22860699 .
- ↑ a b canine vaccines. In: Website Paul Ehrlich Institute . February 13, 2014, accessed March 10, 2014 .
- ↑ Bronchi Shield. In: Website PharmNet.Bund . Retrieved March 11, 2014 .
- ↑ Nobivac BbPi. In: Website PharmNet.Bund . Retrieved March 11, 2014 .
- ^ Rabbit vaccines. In: Website Paul Ehrlich Institute . February 13, 2014, accessed March 11, 2014 .
- ↑ a b c Pig vaccines. In: Website Paul Ehrlich Institute . January 8, 2014, accessed March 30, 2014 .
- ↑ Porcilis AR-T DF - protein dO (non-toxic deletion derivative of Pasteurella multocida dermonecrotic toxin) / inactivated Bordetella bronchiseptica cells. In: European Medicines Agency website . December 20, 2011, accessed March 11, 2014 .
- ↑ Rhiniseng - inactivated Bordetella bronchiseptica, strain 833CER / recombinant type-D Pasteurella multocida toxin (PMTr). In: European Medicines Agency website . September 28, 2010, accessed March 30, 2014 .
- ↑ RESPIPORC ART + EP. In: Website PharmNet.Bund . January 1, 2008, accessed March 30, 2014 .
Web links
- Feline Bordetellosis - The Bordetella bronchiseptica infection of the cat. In: Vetstream clinical reference . Retrieved March 2, 2014 .