Feline Infectious Peritonitis
The feline infectious peritonitis ( FIP ) is a by Feline coronavirus induced infectious disease , the only cats affects (Felidae). The name is derived from the most common clinical manifestation , a peritonitis ( peritonitis from). However, only the pleura can be affected, which is why the name Feline Infectious Polyserositis is rarely used. In addition, a clinical picture without any involvement of the serosa (lining of the body cavities) can occur. If there is a clinical manifestation of the disease, it is usually fatal. In February 2019, trials with a new drug were conducted in California. This drug (GS-441524) is currently only used for research purposes. Therapy with it should have been successful in most cases. The disease occurs worldwide.
Cause and epidemiology
The cause of FIP is a highly virulent coronavirus . The virus, now known as Feline Coronavirus ( FCoV ), was divided into two different viruses by the end of the 1990s: the less pathogenic, so-called " Feline Enteral Coronavirus " (FECV) and the highly pathogenic " Feline Infectious Peritonitis Virus " (FIPV). The latter is only a mutation of the "FECV" within the carrier animal. Both are currently classified as a subtype or isolate of the virus species Alphacoronavirus 1 . The FIPV is antigenetically related to the human coronavirus 229E and the coronaviruses of pigs and dogs. It is not related to the human coronavirus OC43 and the cattle and mouse coronaviruses.
The mutation consists of a deletion in the viral gene 3C, which does not always take place in the same place. The change is favored by the imprecise operation of the viral RNA polymerase , which results in one incorrectly incorporated nucleotide per 1,000 to 10,000 nucleotides during replication . With a total length of the virus RNA of around 30,000 nucleotides, three mutations in the genetic material of the virus per replication are "normal".
FCoV occurs worldwide, but only about five to ten percent of seropositive (infected) domestic cats develop FIP disease. In relation to the entire cat population, FIP has an occurrence frequency ( prevalence ) of one to two percent. Serologically, a distinction is made between two virus types, whereby type 1, which occurs primarily in Europe and the USA, can be reproduced in cell cultures , which is not possible with type 2, which occurs primarily in Japan.
The incubation period is probably up to four months. As early as the second day after infection, the animals excrete the virus in their faeces, nasal secretions and saliva. Virus shedding can last a lifetime. The initially harmless virus is mainly transmitted through contact with infected faeces or contaminated objects. A mouth-to-mouth or mouth-to-nose transmission is also possible. The virus is infectious for up to a week in the vicinity. In addition, humans can transport the virus and pass it on to cats. Virus-carrying cat mothers often infect their fetuses during pregnancy . The transmission of the already mutated form probably does not play a role in the spread of the disease.
In principle, all cat species and age groups are susceptible to FIP. The disease most commonly affects animals between the ages of six months and five years and older animals from 14 years of age. Since cats living in the wild are mostly solitary creatures without permanent droppings, wild animals are much less likely to be infected. Caught feral domestic cats are about 10% seropositive, after a few weeks in an animal shelter, however, almost 90% of the animals. In big cats especially larger stocks are also in zoos endangered leopards are considered particularly susceptible.
Pathogenesis and forms
The pathogenesis of the disease has not yet been fully clarified. The mutation of the initially harmless FCoV variant into the so-called "FIP viruses" takes place in the intestine and can occur years after infection. With the mutation, the virus gains the ability to bind to ribosomes of the phagocytes of the immune system ( monocytes and macrophages ) and to multiply in them ( replication ). As the virus multiplies, the phagocytes break down and the virus particles that are released are taken up by other phagocytes, which causes the virus to spread throughout the body. The release of cell messenger substances activates the cells lining the blood vessels ( endothelial cells ) and thus leads to inflammation . Certain cell messengers also lead to the death of other immune cells such as lymphocytes . The non-mutated variant, on the other hand, multiplies mainly in the intestinal epithelial cells of the jejunum .
Today it is assumed that whether and in what form the disease ultimately occurs depends on the immune status of the individual animal.
In some of the animals the disease does not break out despite the virus mutation due to a strong cell-mediated immune reaction . This enables the immune system to keep the infected blood cells under control. These animals remain without clinical symptoms , but continue to excrete it as latent virus carriers. In some of the animals, complete virus elimination is suspected, but this makes them susceptible to new infections again.
FIP probably only becomes clinically manifest when the immune system is disturbed , e.g. B. through stress or other diseases that lead to a stronger virus replication in the intestine. The formation of antibodies has an influence on the pathogenesis , because they cannot neutralize the virus . With increased antibody formation, more macrophages are also activated, in which the virus continues to multiply. The paradox that the antibodies actually formed to fight the pathogens lead to an aggravation of the disease (" antibody-dependent enhancement of the virus infection " ) is also observed in viral diseases in humans (e.g. AIDS , dengue fever ) . However, this antibody-dependent enhancement probably only plays a role in experimental infections.
In the past, the disease was divided into two main forms (“wet” and “dry”). The boundaries between the two main forms are fluid, however, almost every sick animal shows components of both manifestations, one of which can temporarily dominate. Therefore, this subdivision is increasingly viewed as obsolete in recent literature.
"Wet form"
With a weak cell-mediated immune response , the virus proliferates in the blood ( viraemia ) and the massive formation of immune complexes, activation of the complement system and phagocytes ( macrophages ). This leads to blood vessel inflammation ( vasculitis ) and lymphoplasmacellular perivasculitis ( inflammation in the vicinity of the blood vessels characterized by lymphocytes and plasma cells ) of the serous membranes , which leads to tissue destruction ( necrosis ). However, some authors are of the opinion that the changes are real granulomatous vasculitis and perivasculitis, i.e. an inflammation of the vessels and their surroundings dominated by phagocytes. The lymphoplasmacellular perivasculitis then represents a late stage. Macroscopically, these foci of inflammation appear as whitish nodules. The inflammation also results in the escape of serum and proteins into the body cavities and fibrin deposits on internal organs .
"Dry form"
In the "dry form", larger nodes dominate, which mainly arise within the organs. These are fused foci of inflammation that, like the wet form, arise from vasculitis / perivasculitis. They are sometimes referred to as "granulomatous" changes, but it is not a real granulomatous inflammation . The liquid outlets are not found in this form. It is assumed that this form develops with a less severely weakened cell-mediated immune response and that it represents a milder, more protracted form. It accounts for around 17 percent of FIP cases, but due to the difficult diagnosability (see below), a significant number of unreported cases can be expected.
Symptoms
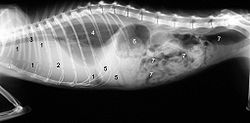
A clinically manifest FIP begins with reduced food consumption ( anorexia ), emaciation and recurring, therapy-resistant fever . The other symptoms depend on the form of expression, with smooth transitions between the two forms can occur. The division into wet and dry forms is, strictly speaking, a description of the macroscopic findings. Microscopically , both forms usually form an identical image.
"Wet form"
The classic "wet form" manifests itself in the accumulation of fluid in the abdominal cavity (dropsy, ascites ) and / or chest cavity ( pleural effusion ). The accumulation of fluid in the abdominal cavity can usually be clinically diagnosed as an increase in circumference with fluctuation. Fluid build-up in the chest cavity can cause severe shortness of breath . A puncture provides a yellowish, stringy, viscous liquid. The fact that this is a protein-rich exudate , which is quite typical in its appearance, is an essential diagnostic criterion.
"Dry form"
The "dry form" manifests itself in nodular changes, especially in the abdomen . The brain , the eyes , the organs of the chest cavity or just the skin can also be affected. Depending on the location of the organ, jaundice , eye diseases ( uveitis , corneal changes ["mutton fat precipitates"], bleeding or fibrin accumulations in the anterior chamber, retinitis ), anemia or neurological symptoms (convulsions, seizures, disorientation, eye tremors , paralysis ) can occur.
diagnosis
An initial clinical suspicion is given in any fever in a younger cat (younger than six years) that does not respond to antibiosis .
Fluid accumulations in the body cavities (“wet form”) and an increased content of globulins in the blood ( hyperglobulinemia ) are already clear indications. Certain changes in the blood count (moderate to severe anemia , neutrophilia, and leukopenia ) are further suspicious factors.
The following further diagnostic test methods are possible:
- Rivalta sample : a puncture removes fluid from an affected body cavity. A drop of glacial acetic acid is added to distilled water in a test tube and a drop of the punctate is added. When infected with FIP, the drop does not dissolve and sinks to the bottom. A negative test result almost certainly rules out FIP ( specificity 98 percent), while a positive test makes it probable, but does not prove it. According to recent studies, the sensitivity is only 52 percent. A positive rival test can also occur in the case of purulent serositis and effusions caused by tumors. The sensitivity is higher in young cats, if you also examine older animals, it drops to 65.5%.
- Antibody detection in the puncture : The detection of antibodies in the puncture by means of antibody staining has a sensitivity and specificity of about 85%.
- Antigen detection in macrophages : In the moist form, a smear can be made from the centrifugate of the puncture and mixed with an anti-coronavirus conjugate . The sensitivity of this detection method is given as 68–95%, depending on the study. The specificity has long been given in the literature as 100%, but a recent study provided three false-positive results in cats with cardiac effusion (specificity 93%).
- Albumin-globulin quotient : Determining the quotient of the albumin and globulin concentration in the blood can also provide an indication of the disease. If the quotient is less than 1, FIP is suspected; values below 0.6 are considered almost diagnostic. However, there are considerable fluctuations in terms of sensitivity and specificity depending on the size of the quotient. With a quotient of 0.9, the sensitivity is 89 percent and the specificity is 76 percent. If the value is below 0.6, the sensitivity is only 48 percent, while the specificity is 99 percent.
- High levels of the acute phase protein alpha1-glycoprotein are indicative of FIP.
- Antibody detection in the blood : A positive indirect antibody detection in the blood is not clear. It only says that the cat had contact with the coronavirus, even if it was only the harmless variant. The sensitivity is 85 percent, but the specificity is only 57 percent. A positive test with a titer of less than 1: 1600 increases the specificity to around 98 percent, but reduces the sensitivity to 33 percent.
- Antigen-antibody complex detection in the blood : The detection of antigen-antibody complexes using ELISA only has a sensitivity of about 50 percent, the specificity is 91 percent.
- FCoV-RT-PCR : Coronavirus RNA and thus viraemia can be detected using an RT-PCR method . When examining blood, the sensitivity is around 15 percent and when examining mononuclear cells it is 29%. The specificity is 86–100%. The detection of virus RNA does not allow a distinction between harmless and mutated coronaviruses. In contrast, RT-PCR from effusion fluid has high sensitivity and specificity (> 90%). The detection in the feces is only used to detect coronavirus shedding, it is unsuitable for diagnosing the disease.
A combination of different procedures increases the diagnostic value. A determination of the lactate dehydrogenase released by hemolysis (an enzyme that converts lactate into pyruvate ) can provide further information about the disease, as can the determination of the increase in the pancreatic enzyme alpha-amylase in cats, usually caused by FIP .
While an antigen detection in the effusion is conclusive, the "dry form" is difficult to detect. Detection methods 4–8 are also possible, but so far only the pathohistological detection is conclusive for the presence of FIP. The immunohistochemical antigen detection on formalin-fixed tissue is more sensitive than on fresh tissue. There are FIP cats without any changes in these parameters, as well as animals that have no FIP despite marked deviations in these parameters. Detection of the antibodies in tissue samples ( biopsy ) from the lungs , liver , kidneys and lymph nodes is considered conclusive, but there are cross-reactions with the harmless FCoV variant and other coronaviruses ( canine coronavirus , TGE virus), to which cats are in principle susceptible , but that do not trigger FIP. A PCR -Virusnachweis in tissues is also commercially available.
Since 2012 there has been a detection method that promises a clear molecular biological characterization of the two coronavirus variants. Mutations in two spike proteins that are directly related to the mutated variant and thus the FIP are detected by means of PCR. This test has also been commercially available since 2013 and can be performed on punctures from effusions, cerebrospinal fluid and aqueous humor as well as on EDTA blood.
Differential diagnosis
With the fairly typical moist form, other causes of ascites and / or pleural effusion must be ruled out. These include, above all, a heart disease , a lack of protein in the blood ( hypoproteinemia ), congestive effusions from tumor diseases , bleeding or bacterial pleurisy or peritonitis; more rarely streptotrichosis (purulent bacterial pleurisy previously thought to be a fungal disease, but the fluid here is brownish-cloudy) or a rupture of the thoracic duct ( chylothorax ). A large part of these diseases can be ruled out quite easily due to the relatively low protein content of the effusion ( transudate ) and the lack of tumor cells or bacteria.
Feline leukemia , feline immunodeficiency syndrome , panleukopenia , lymphosarcoma , yersiniosis, and Tyzzer's disease should be considered if the fever is resistant to therapy and / or nodular changes .
Therapy and prophylaxis
A clinically manifest FIP usually leads to death within a few weeks, especially younger animals with the “moist form” have a short survival time. The mean survival time is nine days, 95% of the infected animals die within one year. So one in 20 cats can live longer than a year, so euthanasia is not immediately indicated.
FIP is curable. In the 2018 study by Niels C. Pedersen, 31 cats were treated with the antiviral GS-441524, a parent nucleoside of GS-5734 that is found to be a low molecular weight antiviral in the prevention of Ebola in rhesus monkeys and the inhibition of coronaviruses Proven to be effective in both tissue culture and mouse infection models. This field trial showed that the chemically less complex 'GS-441524' was highly effective and that it showed promising treatment results in cats with naturally occurring FIP. These results were published in the Journal of Feline Medicine and Surgery (JFMS) on February 13, 2019.
In a study from 2016, a cure with GC376, an inhibitor of the viral 3C-like protease (3CLpro), could also be achieved experimentally in several cases . However, this active ingredient is not yet generally available, so that at the moment only symptomatic therapy combined with immunosuppression is possible. Indications that treatment with feline interferon carried out in addition to immunosuppressive therapy could have a beneficial effect on survival time could not be confirmed in a 2006 study. A therapeutic attempt can be made with high-dose glucocorticoids , possibly in combination with the thromboxane synthase inhibitor ozagrel . An antibiotic is indicated against secondary bacterial infections .
The vaccination against FIP is controversial. The main problem here is that a systemically applied vaccine (vaccine) with the strains used carries the risk of the development of FIP by the vaccine virus, the vaccine virus can be mixed with the field virus and antibody-dependent immune enhancement can occur. The aim of the available vaccine is therefore to generate a local immune response at the cellular level and on the basis of local IgA in the area of the entry portal of the viruses in the nasopharynx. Therefore, the vaccine is instilled into the nose. The only local effect of the vaccine is guaranteed because the vaccine strain can only multiply at a temperature of 31 ° C. The principle of vaccination fails in animals that are already FCoV positive (also due to the harmless variant). It can therefore only be recommended to a limited extent. It is useful for seronegative cats in larger flocks as well as animals kept individually in apartments, which would be overwhelmed in their immune response by accidental contact with introduced virus material (e.g. excrement on the owner's shoes) due to the massive "virus load". The protective effect of the vaccine ( Primucell FIP ® ) produced very different results in clinical studies . Depending on the study, an efficiency between 0 (for no protective effect) and 75 percent was stated.
To prevent the spread of the harmless initial variant of the virus attempt, the concept of "pursuing Early Weaning " (English., Early weaning ), which in 1992 by Addie & Jarrett was presented. Here the pregnant mother cat is isolated from other cats two weeks before the birth and the birth and rearing of young cats are subjected to strict hygiene conditions. At five to six weeks, the kittens are weaned from their mother and separated from her because they are only protected by maternal antibodies up to this point and could then be transmitted from her with the virus. In contrast to successes in Great Britain , in which all kittens were subsequently FCoV-seronegative, this result could not be reproduced in a German study.
A more practicable strategy is to reduce the infection pressure within the cat population. The principle is to thin out the potentially disease-causing FCoV viruses only as much as possible and can already be carried out with simple hygienic methods. The recommended measures are:
- Set up as many excrement boxes as possible, which should be cleaned several times a day
- if possible, always use the same drinking and feeding vessels and clean them daily
- Keep the cats in small groups of 3 to 4 animals
- Removal of strong virus shedders from the group
- Remove dams from the group 2 weeks before the litter and raise the young separately.
history
FIP was observed increasingly in the USA from 1954, although individual reports of suspected FIP cases can be found as early as 1914. In 1963 Jean Holzworth wrote a more detailed paper for the first time, in 1966 Wolfe and Griesemer demonstrated the infectious nature of the disease and gave a more detailed description. In 1968, Zook et al. in cats experimentally infected with the disease, the virus was detected for the first time using an electron microscope. The fact that the pathogen is a coronavirus has been suspected since 1970, but it was not until 1976 that the pathogen was detected ( Osterhaus et al. ) And its reproduction in a cell culture ( Pedersen ). From 1977 the pathogen was initially called "FIP virus" (FIPV). In 1979 the first ELISA test for the detection of antibodies was developed. In 1981 Pedersen et al. the widespread occurrence of the feline enteral coronavirus (FECV) for the first time and showed the great similarity to FIPV. In 1987 Pedersen hypothesized that FECV and FIPV represent a common virus spectrum and differ only in terms of their virulence. In 1998 his working group ( Vennema et al. ) Succeeded in proving that FIPV is only a mutation of FECV. From 2000, the term Felines Coronavirus (FCoV) became the name of the pathogen.
The first experimental attempts at vaccine development go back to the early 1980s. Experiments with different vaccine strains ( heterologous virus, 1979, 1984, 1988; homologous virulent virus, 1983; homologous attenuated virus, 1983; vaccinia virus - recombinants , 1990, 1992) did not produce any detectable protective effect and even led to vaccination diseases, some of which were in the Meaning an antibody-dependent immune enhancement (see below) manifested. The first safe commercial vaccine was manufactured by Pfizer in 1991 .
With the occurrence of Severe Acute Respiratory Syndrome (SARS) and the discovery made in 2003 that the pathogen was a coronavirus, the FCoV and other animal coronaviruses were suspected of being responsible for this severe respiratory disease in humans. The FCoV shows great similarities to the SARS virus in the nucleotide sequence. However, these assumptions have not been confirmed.
literature
- DD Addie, JO Jarrett: A study of naturally occurring feline coronavirus infections in kittens. In: Veterinary Record. Volume 130, 1992, ISSN 0042-4900 , pp. 133-137, doi: 10.1136 / vr.130.7.133 .
- Christina Binder: Comparison of different parameters for the diagnosis of feline infectious peritonitis. Munich 2001 (Munich, university, dissertation, 2001).
- Katrin Hartmann: Feline infectious peritonitis. In: The Veterinary Clinics of North America. Small Animal Practice. Volume 35, No. 1, 2005, ISSN 0195-5616 , pp. 39-79, doi: 10.1016 / j.cvsm.2004.10.011 .
- Katrin Hartmann: Feline infectious peritonitis - diagnosis, treatment and prophylaxis. In: Small Animal Practice. Volume 55, 2010, ISSN 1434-9132 , pp. 561-572.
- Niels C. Pedersen: Virologic and immunologic aspects of feline infectious peritonitis virus infection. In: Advances in Experimental Medicine and Biology. Volume 218, 1987, ISSN 0065-2598 , pp. 529-550, doi : 10.1007 / 978-1-4684-1280-2_69 .
- Harry Vennema, Amy Poland, Janet Foley, Niels C. Pedersen: Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses. In: Virology. Volume 30, No. 1, 1998, ISSN 0042-6822 , pp. 150-157, doi: 10.1006 / viro.1998.9045 .
swell
- ↑ www.fipfree.de .
- ↑ Wilfried Kraft, Ulrich M. Dürr: Cat diseases . Ed .: W. Kraft. 4th edition. M & H Schaper, Hannover 1996, ISBN 3-7944-0178-6 , p. 138 .
- ↑ a b c d e Susanne Held, Reto Neiger: Etiology, epidemiology and diagnosis of feline infectious peritonitis (FIP). In: Small Animal Practice . Volume 58, 2013, pp. 80-96.
- ↑ a b c d Jürgen Kremendahl: Feline infectious peritonitis - a current overview. In: small animal specifically. 2014; 17 (2), pp. 10-14, doi: 10.1055 / s-0033-1361536
- ↑ A. Kipar, H. May, S. Menger, M. Weber, W. Leukert, M. Reinacher: Morphologic Features and Development of Granulomatous Vasculitis in Feline Infectious Peritonitis. In: Veterinary Pathology. Volume 42, No. 3, 2005, ISSN 0300-9858 , pp. 321-330. PMID 15872378 , doi: 10.1354 / vp.42-3-321 .
- ^ Y. Fischer, C. Sauter-Louis, K. Hartmann: Diagnostic accuracy of the Rivalta test for feline infectious peritonitis. In: Veterinary clinical pathology / American Society for Veterinary Clinical Pathology. Volume 41, Number 4, December 2012, pp. 558-567, doi: 10.1111 / j.1939-165X.2012.00464.x , PMID 22913882 .
- ↑ Susanne Held: Accuracy of Diagnostic Tests for Feline Infectious Peritonitis (FIP) in cats with a body cavity effusion. Gießen 2012 (Gießen, University, med. Vet. Dissertation).
- ^ SJ Doenges, K. Weber, R. Dorsch, R. Fux, K. Hartmann: Comparison of real-time reverse transcriptase polymerase chain reaction of peripheral blood mononuclear cells, serum and cell-free body cavity effusion for the diagnosis of feline infectious peritonitis. In: Journal of feline medicine and surgery. Volume 19, Number 4, April 2017, pp. 344-350, doi: 10.1177 / 1098612X15625354 , PMID 26787293 .
- ^ DR Rissi: A retrospective study of the neuropathology and diagnosis of naturally occurring feline infectious peritonitis. In: Journal of veterinary diagnostic investigation: official publication of the American Association of Veterinary Laboratory Diagnosticians, Inc. Volume 30, Number 3, May 2018, pp. 392-399, doi: 10.1177 / 1040638718755833 , PMID 29411701 .
- ↑ Hui-Wen Chang, Herman F. Egberink, Rebecca Halpin, David J. Spiro, Peter JM Rottier: Spike protein fusion peptide and feline coronavirus virulence. In: Emerging Infectious Diseases . Volume 18, No. 7, July 2012, ISSN 1080-6059 , pp. 1089-1095, doi: 10.3201 / eid1807.120143 , PMID 22709821 , PMC 3376813 (free full text).
- ↑ Deutsches Tierärzteblatt. Volume 61, 2013 ISSN 0340-1898 , p. 281.
- ↑ Joachim Frey : Diseases of the respiratory organs. In: Ludwig Heilmeyer (ed.): Textbook of internal medicine. Springer-Verlag, Berlin / Göttingen / Heidelberg 1955; 2nd edition ibid. 1961, pp. 599-746, here: p. 694 ( streptotrichosis ).
- ↑ Marian C. Horzinek : Can Feline Infectious Peritonitis (FIP) be controlled? In: Kleintiermedizin No. 5, 2016, pp. 210–217.
- ↑ Pedersen NC, Perron M, Bannasch M, et al. Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis. J Feline Med Surg. 2019; 21 (4): 271-281. doi: 10.1177 / 1098612X19825701
- ↑ Kim Yunjeong et al .: Reversal of the Progression of Fatal Coronavirus Infection in Cats by a Broad-Spectrum Coronavirus Protease Inhibitor. In: PLOS pathogens 2016, doi: 10.1371 / journal.ppat.1005531
- ↑ Susanne Ritz, Herman Egberink, Katrin Hartmann: Effect of Feline Interferon-Omega on the Survival Time and Quality of Life of Cats with Feline Infectious Peritonitis. In: Journal of Veterinary Internal Medicine. Volume 21, No. 6, 2006, ISSN 0891-6640 , pp. 1193-1197, doi: 10.1111 / j.1939-1676.2007.tb01937.x .
- ↑ DD Addie and JO Jarrett: A study of naturally occurring feline coronavirus infections in kittens. In: Veterinary Record. Volume 130, 1992, ISSN 0042-4900 , pp. 133-137, doi: 10.1136 / vr.130.7.133 .
- ^ John Stavrinides, David S. Guttman: Mosaic evolution of the severe acute respiratory syndrome coronavirus. In: Journal of Virology. Volume 78, No. 1, 2004, ISSN 0022-538X , pp. 76-82. PMID 14671089 , doi: 10.1128 / JVI.78.1.76-82.2004 .
Web links







