Anthrax toxin
Anthrax toxin (also: anthrax toxin ) is a mixture of proteins that the anthrax - pathogen , the bacterium Bacillus anthracis is produced and which is responsible for the danger of anthrax infection. It is excreted by the bacterium during infection , penetrates the host's cells and causes their destruction.
The subunits of anthrax toxin are called protective antigen (PA), lethal factor (LF) and edema factor (EF). The forming of the anthrax toxin protein complexes belong to the large group of Bacillus - and Clostridium - exotoxins , which consists of two functionally complementary subunits are constructed ( AB toxins ): PA + LF is the lethal toxin and PA + EF the Ödemtoxin .
For structural biology , anthrax toxin is a particularly accessible model for studying the mechanisms involved in the transport of large proteins to the transmembrane . Although the details of the anthrax toxin poisoning process have been extensively studied, not all questions have been answered due to the complexity of the poisoning process. Nevertheless, in addition to antibiotics, several vaccinations and highly effective antibodies are available for treatment.
history
Anthrax was the first disease in which the cause of infection with microorganisms could be proven beyond doubt. Robert Koch not only succeeded in multiplying the pathogen ( Bacillus anthracis ) in the laboratory in 1876 , he also demonstrated its spore formation and was able to make healthy animals sick with it. This first evidence of a transmission path initiated the so-called golden age of microbiology . Only a few years later, Louis Pasteur developed a vaccine for sheep after he had previously demonstrated the principle of weakening virulence ( attenuation ) through culture at elevated temperatures using fowl cholera bacteria ( Pasteurella multocida ) . The historical field tests took place in Pouilly-le-Fort in 1881 .
The amount and quality of publications on anthrax toxin from 1953 to 1968 is remarkable, in contrast to the almost complete lack of publications between 1969 and the early 1980s. In 1954 it was shown for the first time that the blood serum of infected guinea pigs, which were treated late with antibiotics , contained toxins. In the years up to 1962 British and American researchers developed the methods for the isolation and purification of the subunits LF, EF and PA. When it became clear that the edema toxin (see below) is not fatal, the focus was exclusively on investigating the lethal toxin. The discovery in 1962 that a special strain of laboratory rats , the so-called Fischer rats, is highly sensitive to the toxin, is still used today to measure the purity of a toxin preparation.
Poisoning process
If the living conditions for B. anthracis are favorable, bacteria form again from the endospores ; this happens, for example, on the skin, in the lungs or in the intestines of vertebrates , whereby the proteins LF, PA and EF are produced and secreted in large quantities . Outside the bacterium, a protease shortens the PA to PA-63 and this forms a prepore on the host cell that can bind a maximum of four molecules of LF and / or EF. After the toxin has been introduced into the host cell, it is part of an endosome that matures into a lysosome within minutes , exposing the proteins inside to a strong acidification. This leads to the formation of a “clamp” in the prepore, which turns the ring into a pore-forming toxin , which penetrates the membrane and via which the bound LF and EF units reach the cytosol.
If LF and EF are free in the cytosol, their disruptive functions cannot be stopped. Due to their enzyme properties , they are not used up in the process. LF is a protease that intervenes in particular in the MAPKK signaling pathway , while EF, as an adenylyl cyclase, produces an excess of cAMP and causes the overexpression of the anthrax toxin receptors . The escalation caused by large amounts of toxin leads to cell death.
Since all cells are sensitive to anthrax toxin and the death of the cells of the innate immune system favors the infection, increasing amounts of interleukins are released as part of the immune response . The resulting shock with respiratory and heart failure is ultimately the cause of death for the host. If the toxin is applied directly, the clinical picture is different from an infection. The ultimate cause of death here is not systemic shock, but rather the destruction of blood vessels. In addition, individual vertebrate species ( rodents , frogs ) are particularly sensitive to the toxin and can die from it within a few hours, while a lethal infection can take days.
The subunits PA, LF, EF
The pathogenicity of the anthrax bacterium is mainly linked to the presence of the two plasmids pXO1 and pXO2, which weigh 110 and 60 megadaltons . While pXO2 codes , among other things, for the polyglutamate envelope of the bacterium , pXO1 contains the genetic code for the toxin subunits as part of a so-called pathogenicity island , which extends between positions 117177 and 162013 (total in plasmid 181654 base pairs ) and contains eight identified genes. Three of them, the genes pagA, lef and cya code for the proteins PA, LF and EF. As soon as a threshold value of carboxylic acids or carbon dioxide is exceeded, the transcription factor atxA (part of a still unknown two-component system ) reacts and starts the expression of the pathogenicity factors toxin plus polyglutamate. The subunits PA, LF and EF are distributed in a ratio of 20: 5: 1.
Protective antigen (PA)
| Protective antigen (PA-63) | ||
|---|---|---|

|
||
| Ribbon model of PA-83, the individual domains colored, calcium as red spheres: yellow. PA-20, will be cut off. Blue. Calcium and LF / EF binding. Green. Membrane domain. Orange. Oligomerization interface. Pink. Receptor binding (according to PDB 1ACC ) | ||
| Mass / length primary structure | 568 aa | |
| Secondary to quaternary structure | Homooctamer | |
| Cofactor | Ca ++ | |
| Precursor | prepro-PA (764 aa) PA-83 (735 aa) |
|
| Identifier | ||
| Gene name (s) | pagA | |
| External IDs | ||
| Transporter classification | ||
| TCDB | 1.C.42 | |
| designation | BAPA family | |


The protective antigen (PA) is a protein that is excreted by B. anthracis in a length of 735 amino acids . In the shortened form ( PA-63 ; 568 amino acids) it is part of the anthrax toxin and is combined with seven other PA-63 units to form an eight-part prepore. This prepore binds one or more of the LF / EF subunits, causes the introduction into a host cell by means of endocytosis by binding to a host receptor , and finally transforms into a fully grown pore within the endosome , which causes the LF / EF subunits to be discharged from the endosome inside the cell (cytosol). This transport function of PA-63 is essential for the pathogenicity of the bacterium.
The transport equation of the pore is:
- LF or EF (endosome interior) LF or EF (cytosol)
Expression, modification, oligomerization
The pagA gene (2294 base pairs ) positioned on the plasmid pXO1 codes for a 764 amino acid long precursor protein which contains a 29 amino acid long signal sequence at the start of translation . The Sec transport system in the cell membrane of the bacterium recognizes this signal sequence and then triggers the secretion of the still unfolded protein, whereby the signal sequence is separated. The extracellular chaperone prsA helps the now 735 amino acid long PA-83 to fold into its final shape.
There are contradicting results as to where exactly in the host the PA-83 is abbreviated to PA-63. In each case, a protease cleaves an additional 167 amino acids from PA-83. Only this shortening exposes the protein domains that allow the LF and EF factors to bind to PA-63. And only now is PA-63 able to assemble with several identical units to form a ring-shaped prepore . Although it is still unclear whether the preferred form of PA-63 is the heptamer or the octamer, the octamer appears to be more stable than the heptamer under the conditions prevailing during infection. Nevertheless, both forms can induce endocytosis at host cell receptors.
Binding to host receptors
Three receptor proteins, all of which are found on vertebrate cells, have been shown to allow anthrax toxin to enter the cell. It is the anthrax toxin receptor 1 (ATR, also: TEM8), which normally binds type 1 collagens ; the anthrax toxin receptor 2 (ATR2, also: CMG-2), which has laminin and type 4 collagens as natural ligands ; and heterodimeric integrin -β 1 . All of these receptors contain a so-called integrin I or Von Willebrand A domain, the amino acids of which complex a divalent metal ion (Ca ++ , Mn ++ , Mg ++ ) on the receptor surface, creating a metal ion Form dependent adhesive point (MIDAS, from English metal-ion dependent adhesion site ). At this point at the latest, when bound to the receptor, a protease cuts the PA-83 and oligomerizes the resulting PA-63 into a seven or eight-part prepore, which leads to an accumulation of just as many receptors in the cell membrane. In turn, up to four LF or EF units are bound to the prepore. Endocytosis of the entire complex, which now contains up to 25 subunits, takes place via cholesterol-rich patches of the membrane, the lipid rafts , and is supported by clathrin . The crystallographic data give the dimensions of the prepore: diameter 160 Å , height 85 Å and an internal diameter of about 35 Å.
Configuration change to the pore
Due to the general difficulty of studying membrane transport proteins by X-ray crystallography , little is known about the structure of pores and their formation and transport mechanism. In this regard, PA-63 is one of the best-studied transport proteins for the transport of large proteins. First, Blaustein and colleagues demonstrated that PA-63 forms a channel in artificial membranes under acidic conditions that was permeable to ions . Later studies with ions of different sizes gave a value of 12 Å for the inner diameter, very similar to that of other A / B toxins such as botulinum , diphtheria or tetanus toxin . As soon as the pore transported one of the factors LF or EF, it was no longer permeable to ions.
In 2004, Tam Luong Nguyen succeeded in postulating a plausible mechanism for the formation of the PA-63 pore by comparing it with the α-hemolysin of Staphylococcus aureus and in creating a spatial model of the pore that worked well with all the results available to date agreed. A protein domain is essential here, the backbone of which forms a meander motif that, in an acidic environment, folds outwards like a bracket and forms a beta sheet . Seven or eight of these leaflets are formed into a tube ( beta barrel ) that has exactly the required properties. In a landmark study could Hiroo Katayama and employees in 2008 show that the chaperone GroEL is suitable to stabilize the PA-63 pore so that electron microscopic measurements can be performed. In retrospect, these confirmed the assumptions of the Nguyen model.
Translocation of LF and EF
The mechanism by which the virulence factors are transported out of the endosome is also not yet fully understood. Great progress has been made through the use of electrophysiological measurement systems that were developed by several research teams from 2004 onwards. These consist of two with potassium chloride solution filled chambers on a planar phospholipid - double membrane are connected. Electrical voltages and pH gradients are easily adjustable and measurable. In this way, the transportability of PA-63 pores or factors that have been specifically modified in individual amino acids can be measured under realistic conditions and thus it can be determined which amino acids actually take part in the process. On the basis of such experiments, various research groups were able to prove from 1994 onwards that LF with the N terminus is first transported through the pore; that the electrical potential in the direction of transport is positive; that when LF is in the pore, no more particles can get through; that a certain amino acid, phenylalanine-427, with its six to seven identical neighbors inside the beta-barrel forms a constriction in the tube and the active center of the transport function. This constriction was named φ terminal (Engl. Φ-clamp ), it interacts directly with the transported protein.
In 2006, Bryan A. Krantz finally proposed a model for the transport mechanism of the PA-63 pore on the basis of the results so far, in which the factor to be transported almost unfolds due to the acidic environment, i.e. is present as a linear chain, which only with the help of the pH- Gradient and Brownian molecular motion, a net motion out of the endosome. The assumption of this principle, known as the “ molecular ratchet ”, has not yet been refuted.
Lethal factor (LF)
| Lethal factor | ||
|---|---|---|
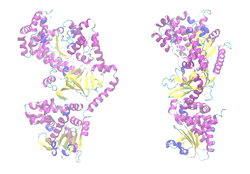
|
||
| Ribbon model of the LF monomer, zinc as a ball, according to PDB 1J7N | ||
|
Existing structural data: s. UniProt |
||
| Mass / length primary structure | 776 amino acids | |
| Cofactor | Zn ++ | |
| Precursor | prepro-LF (809 aa) | |
| Identifier | ||
| Gene name (s) | lef | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 3.4.24.83 , metallopeptidase | |
| MEROPS | M34.001 | |
| Response type | Hydrolysis of peptide bonds | |
| Substrate | all MAP2K except MAP2K5 | |
| Products | Waste proteins | |
The lethal factor (LF) is an enzyme from the family of neutral zinc metallopeptidases . It specifically catalyzes the hydrolysis of peptide bonds in certain enzymes in the host cell, the MAP kinase kinases (MAPKK, MAP2K, MEK). It is an endopeptidase .
Particularly cell-toxic effects on all cells were observed when the lethal toxin was administered. Due to their similarity to the mode of action of artificial MEK inhibitors, these cytotoxic effects can also be attributed to the inhibition of the MEK signaling pathways by the lethal factor.
The catalyzed reaction is:
- MEK + H 2 O protein fragments
The enzyme function only comes about in the presence of the cofactor zinc.
The lethal factor is also initially synthesized with a signal sequence that is cut off after export via the Sec system.
Edema factor (EF)
| Edema factor | ||
|---|---|---|

|
||
| Ribbon model of the EF (blue) in a complex with calmodulin (gray), deoxy-ATP, Ca and Mg as spherical caps , according to PDB 1XVF . The lower domain binds to PA-63. | ||
| Mass / length primary structure | 767 amino acids | |
| Cofactor | Ca ++ , Mg ++ , calmodulin | |
| Precursor | (800 aa) | |
| Identifier | ||
| Gene name (s) | cya | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 4.6.1.1 , lyase | |
| Response type | Ring closure | |
| Substrate | ATP | |
| Products | 3 ', 5'-cAMP + PP i | |
The edema factor (EF, also more precisely: calmodulinsensitive adenylyl cyclase ) from B. anthracis is an enzyme that catalyzes the conversion of ATP into cAMP . It is a class II adenylyl cyclase . The uninhibited production of the unspecific second messenger cAMP triggers further signals, depending on the cell type, whose overall effect is difficult to overlook. However, there has recently been evidence that EF is responsible for damage to the immune system and blood vessels, contributing to the overall lethality of the infection. EF alone has a fatal effect on the cell or the entire organism only in high doses.
The catalyzed equilibrium is:
- ATP cAMP + PP i
For a functioning enzyme it is necessary to complex it with a calcium and magnesium ion , as well as a calmodulin molecule .
The edema factor is also initially synthesized with a signal sequence that is cut off after export via the Sec system.
evolution

The evolution of anthrax toxin is the sum of the evolution of its protein subunits . Although the pathogenicity operon was presumably transposed onto the plasmid pOX1 as a whole (so-called pathogenicity island ) , this event is older than the differentiation of the genera Bacillus and Clostridium from their common predecessor. The reason is that several species of these genera produce proteins that are homologous to the subunits PA, LF and EF of anthrax toxin . In addition, LF and EF are also homologous to each other, so that an enzyme + PA double toxin can be considered as a common ancestor . This resulted in the naming of the group as A / B toxins.
Structural biology databases have developed amino acid patterns that match all such related proteins. This means that it is now possible to identify and mark corresponding genes immediately after genome sequencing ( gene annotation ). With patterns and tools like the BLAST algorithm , it is easy to show that the A subunits (the enzymes) are more conserved than the respective PA because they are more similar to one another.
Protection options
Anthrax infection is treatable with antibiotics . However, this is often not enough to avoid damage from the released toxin. To protect livestock and humans from the effects of an anthrax infection, special vaccines and antidotes have been established. Also, the sensitivity to the toxin is not the same in all people.
By the United States Department of Homeland Security -funded research projects on a large scale have led to a series of products that the land in the Strategic National Stockpile available. Despite the rarity of possible applications, enough data is available to demonstrate the efficiency of tailored human monoclonal antibodies against the anthrax toxin subunits. They can now be used in cases that no longer respond to antibiotics.
vaccination
The vaccination method developed by Pasteur for livestock consisted of two inoculations at an interval of two weeks: cells from B. anthracis cultures that were attenuated for different lengths of time at 42 to 43 degrees . Such a treatment removes the plasmid pOX1 from almost all cells and it is assumed that the following subclinical infection leads to the success of the vaccination, since pOX1-free cells have no effect. The Pasteur vaccine was replaced in the 1930s by Carbozoo , a vaccine consisting of bacterial spores in a ten percent saponin solution. From this, the star vaccine , which is still used today, developed from spores of the strain 34F 2 in a 0.5% saponin solution.
In the course of the Cold War , the great powers developed vaccines for humans that are commercially available today: the Russian vaccine is based on a modification of the star stem and is applied by means of scarification . Its effectiveness is unknown, in contrast to the high number of side effects and contraindications . The vaccine developed by the USA ( Anthrax Vaccine Absorbed , AVA ) is marketed by Emergent BioSolutions under the trade name BioThrax ; it consists of PA produced in fermenters on an aluminum hydroxide matrix. The British product ( Anthrax Vaccine Precipitated , AVP ) differs only slightly from it in that it has a higher LF and EF content. AVA and AVP are used prophylactically by the military .
After the attacks of September 11, 2001 and the subsequent dispatch of anthrax spores , the need for a population-wide vaccine that the military vaccines AVA and AVP could not satisfy increased: in particular, the frequency of the necessary application (6 injections over 18 months, then once a year ) is unacceptable for such use. In the research that followed, all known methods of vaccine production were tried. As with many other bacterial vaccine production projects, however , clinical trials have remained in short supply. For this reason, and also because of the easy treatment with antibiotics and human antibodies, high hurdles are set for new vaccines. With SparVax , a vaccine made from recombinant, modified PA, the company PharmAthene has developed a product that can be manufactured with high purity and better stored. In the next generation, the number of injections required is to be further reduced.
The acellular vaccine BioThrax®, whose effect is based on the induction of antibodies against the protective antigen, has been approved by the Paul Ehrlich Institute since 2013 . This enables the active immunization of persons exposed to anthrax pathogens, such as veterinarians and skinners.
The vaccination of animals to protect against anthrax is prohibited in Germany. Vaccines for humans are not freely available and not approved in Germany.
Antidote
Human monoclonal antibodies (humAB) against the PA subunit in anthrax toxin are the basis of several products that were developed for use as an antitoxin. You can still neutralize the deadly effects of the toxin days after you become infected with anthrax spores. Other small molecules with similar effects have been discovered, but no clinical experience is available on them.
The humAB Thravixa developed by emergent BioSolutions is in phase 1 of approval. PharmAthene and Medarex are already on the market with their humAB Valortim , which is approved as an orphan drug , and its further development has been promoted. The high-affinity HuMAb Anthim of Elusys , the equally from the FDA Orphan Status received, this company brought already a 140-million-dollar contract. It can be assumed that it is not only the companies mentioned that have other anthrax antitoxins in the pipeline .
When looking for low molecular weight compounds that can be used as anthrax antitoxins, there are naturally three main approaches: the inhibition of the PA transport function and the inhibition of the LF and EF enzyme functions. The inhibition of the transport function can also have different approaches: the presentation of a receptor without endocytosis, receptor antagonism , as well as several possibilities to inhibit the formation of the pore and its function. A large number of substances have therefore already been found. Medicinal substances with originally different indications include statins , neomycin B and verapamil .
Natural gene variations
The International HapMap Project analyzes human genes and their variations worldwide ; cell lines are also recorded. In 2011 , Martchenko et al. Were able to use lymphoblasts from 234 people to demonstrate that the sensitivity of these cells to PA-63 varied over a wide range and that this sensitivity correlates with the amount of mRNA produced that is responsible for the anthrax toxin receptor 2 (CMG-2 ) coded. The study showed that CMG-2 expression fluctuates over four orders of magnitude in different people and that cells from three Europeans are even insensitive to anthrax toxin. The inheritance of the relative insensitivity could be demonstrated; the distribution of the values indicates the polygeny of this phenotype .
Individual evidence
- ^ A b David P. Clark, Nanette J. Pazdernik: Molecular Biotechnology: Fundamentals and Applications . Springer, 2009, ISBN 3-8274-2128-4 , pp. 563 ff .
- ↑ InterPro : IPR014781 Anthrax toxin, lethal / endema factor, N- / C-terminal (English)
- ↑ a b H. Katayama, BE Janowiak et al: GroEL as a molecular scaffold for structural analysis of the anthrax toxin pore. In: Nature structural & molecular biology . Volume 15, Number 7, July 2008, pp. 754-760, doi: 10.1038 / nsmb.1442 . PMID 18568038 . PMC 2504863 (free full text).
- ↑ R. Koch: Studies on bacteria: V. The etiology of anthrax disease, based on the history of the development of Bacillus anthracis. (PDF; 11.1 MB). In: Contributions to the biology of plants. 2 (2), 1876, pp. 277-310. Cohns
- ^ L. Pasteur, Chamberland and Roux: De l'attenuation des virus et de leur retour à la virulence. In: Comptes rendus. 92, 1881, p. 492
- ↑ P. Mikesell, BE Ivins et al.: Plasmids, Pasteur, and Anthrax. (PDF; 199 kB) In: ASM News. 49, 1983, p. 320
- ↑ a b c d e f M. Moayeri, SH Leppla: Cellular and systemic effects of anthrax lethal toxin and edema toxin. In: Molecular Aspects of Medicine . Volume 30, Number 6, December 2009, pp. 439-455. doi: 10.1016 / j.mam.2009.07.003 . PMID 19638283 . PMC 2784088 (free full text). (Review).
- ↑ H. Smith, J. Keppie: Observations on experimental anthrax; Demonstration of a specific lethal factor produced in vivo by Bacillus anthracis. In: Nature . Volume 173, Number 4410, May 1954, pp. 869-870. PMID 13165673 .
- ↑ FA Beall, MJ Taylor, CB Thorne: Rapid lethal effect in rats of a third component found upon fractionating the toxin of Bacillus anthracis. In: Journal of bacteriology. Volume 83, June 1962, pp. 1274-1280. PMID 13866126 . PMC 279445 (free full text).
- ^ A b G. van der Goot, JA Young: Receptors of anthrax toxin and cell entry. In: Molecular aspects of medicine. Volume 30, Number 6, December 2009, pp. 406-412, doi: 10.1016 / j.mam.2009.08.007 . PMID 19732789 . PMC 2783407 (free full text). (Review).
- ↑ a b R. J. Collier: Membrane translocation by anthrax toxin. In: Molecular Aspects of Medicine. Volume 30, Number 6, December 2009, pp. 413-422, doi: 10.1016 / j.mam.2009.06.003 . PMID 19563824 . PMC 2783560 (free full text). (Review).
- ↑ RT Okinaka, K. Cloud et al: Sequence and organization of pXO1, the large Bacillus anthracis plasmid harboring the anthrax toxin genes. In: Journal of bacteriology. Volume 181, Number 20, October 1999, pp. 6509-6515. PMID 10515943 . PMC 103788 (free full text).
- ↑ a b c d R. Bhatnagar, S. Batra: Anthrax toxin. In: Critical reviews in microbiology. Volume 27, number 3, 2001, pp. 167-200, doi: 10.1080 / 20014091096738 . PMID 11596878 . (Review).
- ↑ a b c J. G. Bann: Anthrax toxin protective antigen - insights into molecular switching from prepore to pore. In: Protein science: a publication of the Protein Society. Volume 21, Number 1, January 2012, pp. 1-12. doi: 10.1002 / pro.752 . PMID 22095644 .
- ^ RA Pimental, KA Christensen u. a .: Anthrax toxin complexes: heptameric protective antigen can bind lethal factor and edema factor simultaneously. In: Biochemical and biophysical research communications . Volume 322, Number 1, September 2004, pp. 258-262, doi: 10.1016 / j.bbrc.2004.07.105 . PMID 15313199 .
- ↑ TL Nguyen: Three-dimensional model of the pore form of anthrax protective antigen. Structure and biological implications. In: Journal of biomolecular structure & dynamics. Volume 22, Number 3, December 2004, pp. 253-265. PMID 15473701 .
- ↑ UniProt P09616
- ↑ AD Pannifer, TY Wong include: Crystal structure of the anthrax lethal factor. In: Nature. Volume 414, Number 6860, November 2001, pp. 229-233, doi: 10.1038 / n35101998 . PMID 11700563 .
- ↑ FJ Maldonado-Arocho, JA Fulcher u. a .: Anthrax edema toxin induces anthrax toxin receptor expression in monocyte-derived cells. In: Molecular microbiology. Volume 61, Number 2, July 2006, pp. 324-337, doi: 10.1111 / j.1365-2958.2006.05232.x . PMID 16856939 .
- ^ WJ Tang, Q. Guo: The adenylyl cyclase activity of anthrax edema factor. In: Molecular aspects of medicine. Volume 30, number 6, December 2009, pp. 423-430, doi: 10.1016 / j.mam.2009.06.001 . PMID 19560485 . PMC 2783455 (free full text). (Review).
- ↑ S. Perelle, M. Gibert et al: Characterization of Clostridium perfringens iota-toxin genes and expression in Escherichia coli. In: Infection and Immunity . Volume 61, Number 12, December 1993, pp. 5147-5156. PMID 8225592 . PMC 281295 (free full text).
- ↑ K. Kimura, T. Kubota et al: The gene for component-II of botulinum C2 toxin. In: Veterinary microbiology. Volume 62, Number 1, April 1998, pp. 27-34. PMID 9659689 .
- ↑ S. Stubbs, M. Rupnik et al: Production of actin-specific ADP-ribosyltransferase (binary toxin) by strains of Clostridium difficile. In: FEMS microbiology letters. Volume 186, Number 2, May 2000, pp. 307-312. PMID 10802189 .
- ↑ InterPro : IPR003541 Anthrax toxin, lethal / endema factor (English)
- ↑ InterPro : IPR003896 Bacterial exotoxin B (English)
- ^ A b R. J. Cybulski, P. Sanz, AD O'Brien: Anthrax vaccination strategies. In: Molecular aspects of medicine. Volume 30, number 6, December 2009, pp. 490-502, doi: 10.1016 / j.mam.2009.08.006 . PMID 19729034 . PMC 2783700 (free full text). (Review).
- ↑ web presence of emergent
- ↑ T. Chitlaru, Z. Altboum et al .: Progress and novel strategies in vaccine development and treatment of anthrax. In: Immunological Reviews . Volume 239, Number 1, January 2011, pp. 221-236, doi: 10.1111 / j.1600-065X.2010.00969.x . PMID 21198675 . (Review).
- ↑ PharmAthene website
- ↑ pei.de ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice.
- ↑ a b Y. Li, K. Sherer et al.: New insights into the pathogenesis and treatment of anthrax toxin-induced shock. In: Expert opinion on biological therapy. Volume 7, Number 6, June 2007, pp. 843-854, doi: 10.1517 / 14712598.7.6.843 . PMID 17555370 . (Review).
- ↑ K. Desai: Bioterrorism: Anthrax and Biotech Companies. In: seekingalpha.com , Ed .: Seeking Alpha. Jun 17, 2008. Retrieved on: Feb 20, 2012.
- ↑ M. Forino, S. Johnson et al.: Efficient synthetic inhibitors of anthrax lethal factor. In: Proceedings of the National Academy of Sciences . Volume 102, Number 27, July 2005, pp. 9499-9504, doi: 10.1073 / pnas.0502733102 . PMID 15983377 . PMC 1160517 (free full text).
- ↑ AM deCathelineau, GM Bokoch: Inactivation of rho GTPases by statins attenuates anthrax lethal toxin activity. In: Infection and Immunity. Volume 77, Number 1, January 2009, pp. 348-359, doi: 10.1128 / IAI.01005-08 . PMID 18936176 . PMC 2612271 (free full text).
- ↑ M. Martchenko, SI Candille et al. a .: Human genetic variation altering anthrax toxin sensitivity. In: Proceedings of the National Academy of Sciences . Volume 109, Number 8, February 2012, pp. 2972-2977, doi: 10.1073 / pnas.1121006109 . PMID 22315420 . PMC 3286947 (free full text).
Web links
- D. Goodsell: PDB Molecule of the Month 101: Anthrax Toxin. doi : 10.2210 / rcsb_pdb / mom_2002_4



