Polyvinyl chloride
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|

|
|||||||
| General | |||||||
| Surname | Polyvinyl chloride | ||||||
| other names |
|
||||||
| CAS number | 9002-86-2 | ||||||
| Monomer | Vinyl chloride | ||||||
| Molecular formula of the repeating unit | C 2 H 3 Cl | ||||||
| Molar mass of the repeating unit | 62.50 g mol −1 | ||||||
| Type of polymer | |||||||
| Brief description |
White dust |
||||||
| properties | |||||||
| Physical state |
firmly |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Polyvinyl chloride ( abbreviation PVC ) is a thermoplastic polymer that is produced from the monomer vinyl chloride by chain polymerization . PVC is the third most important polymer for plastics after polyethylene and polypropylene .
The PVC plastics are divided into hard and soft PVC. Rigid PVC is used, for example, to manufacture window profiles, pipes and records . Soft PVC contains plasticisers that make the material elastic. It is used, for example, for cable sheathing and floor coverings .
history
The French chemist Henri Victor Regnault was the first to produce vinyl chloride in Justus von Liebig's laboratory in Giessen in 1835 and noticed that a white powder - polyvinyl chloride - formed from it after prolonged exposure to sunlight , but could not understand the significance of his discovery.
In 1912 Fritz Klatte developed the synthesis of vinyl chloride from ethyne and hydrogen chloride at the Griesheim-Elektron chemical factory . He too exposed glass vessels containing vinyl chloride and various additives to sunlight. In doing so, he laid the foundations for the manufacture of PVC. In 1913 Klatte patented the "polymerisation of vinyl chloride and use as a horn substitute, as films, synthetic threads and for paints". However, marketable products were not developed.
With the scarcity of raw materials during and after the First World War , efforts were intensified to use PVC as a raw material in order to replace expensive raw materials with inexpensive materials. However, it did not find further applications until the late 1920s. In 1928 the large-scale expansion took place through production in the USA and in 1930 in Rheinfelden (Baden) by BASF ; In 1935 IG Farben started producing PVC.
In 1935, the production of soft PVC succeeded in Bitterfeld (D). Igelit was a trademark of that time . The first PVC products were foils and pipes. The latter were relocated to Bitterfeld and Salzgitter (D) in 1935 . After 1945, PVC was the most widely produced plastic in the world. In 1948 vinyl records were finally made, which finally replaced shellac .
With the growth of the chemical industry, caustic soda was required in ever larger quantities. Sodium hydroxide is obtained from sodium chloride by means of chlor-alkali electrolysis . The by-product is chlorine . The development of chlorine chemistry is based, among other things, on the cost-effective by-product, which also favored the production and marketing of PVC. Critics regard PVC as a “chlorine sink” for the by-product of chlor-alkali electrolysis.
In the United States, the material was further developed in the 1960s to post-chlorinated PVC (chlorinated polyvinyl chloride), which is abbreviated to "PVC-C" according to DIN, and to "CPVC" abroad. The mass fraction of chlorine in PVC-C is above the 56.7% of PVC and can be up to 74%. At higher temperatures it is more resistant to corrosion and has better mechanical properties than PVC, so that it is also suitable for the manufacture of pipes for hot water supply and, with restrictions, even for heating circuits. Trade names are, for example, Lucalor, Corzan, Trovidur and (formerly) Glastoferan.
Manufacturing
Polyvinyl chloride is produced by radical chain polymerisation from the monomer vinyl chloride (H 2 C = CHCl):
Essentially, three different polymerization processes are common. The tacticity of the repeat units is mainly atactic in all procedures. The up to 10% crystalline fraction of the polymer has a syndiotactic structure.
E-PVC
The oldest process is emulsion polymerization (first introduced in 1929). So-called E-PVC is obtained. With the help of emulsifiers , vinyl chloride is stirred into water as small droplets. Hydrogen peroxide or potassium peroxodisulfate , for example, are used as water-soluble initiators . At elevated temperatures, polyvinyl chloride particles form from the monomer droplets. The unreacted monomer is withdrawn from these primary particles under reduced pressure. The emulsifiers used remain in the product. The process can be carried out either continuously or batchwise. Polymer dispersions from this process are used, among other things, for adhesives or coating agents.
S-PVC
Vinyl chloride is liquefied under pressure in an autoclave and water is added. Vigorous stirring creates a suspension of very small vinyl chloride droplets in water. Organic peroxides soluble in the monomer or certain aliphatic azo compounds, such as, for example, azobis (isobutyronitrile) (AIBN), are used as the polymerization initiator . It is a suspension polymerization and the resulting product is called S-PVC.
Protective colloids are added in very small amounts in order to prevent the droplets from sticking together during the course of the polymerization. The grains are degassed to remove unreacted monomers and water. Around 90% of PVC production takes place this way.
M-PVC
In bulk polymerization, the polymerization is carried out directly in liquid vinyl chloride with an initiator that is soluble in it, usually an organic peroxide. The product is called M-PVC. The conversion is only carried out to about 80% and the unreacted monomer is removed under reduced pressure. Compared to E- and S-PVC, M-PVC is very pure. The closely distributed grain size is approx. 100 µm. M-PVC is preferred for applications in which a high level of transparency is required. The same applies to sterilization films.
Hard PVC and soft PVC
PVC is divided into hard PVC (abbreviation PVC-U, where U stands for unplasticized ) and soft PVC (abbreviation PVC-P, where P stands for plasticized ). Pipes and profiles, for example for windows and pharmaceutical films, are made from rigid PVC. Soft PVC plays an important role as a cable insulator and is also used in floor coverings, hoses, shoe soles and roof seals. Soft PVC contains up to 40 percent plasticizers ; Rigid PVC basically does not contain any plasticizers.
Additives
The inherently brittle and hard PVC is adapted to the most diverse areas of application with additives , primarily stabilizers and impact modifiers . The additives improve the physical properties such as the temperature, light and weather resistance, the toughness and elasticity, the notched impact strength , the gloss and serve to improve the processability. The additives should have a high effect in the lowest possible concentration, should not impair the manufacturing processes for the plastic molded part and give the product the desired useful life. Acrylate polymers or chlorinated polyethylene are generally used as impact modifiers. The processing of PVC is also improved by modifiers, so a faster plasticization of PVC is achieved.
PVC is a thermoplastic polymer that is processed in the temperature range from 160 to 200 ° C. At these temperatures a decomposition process begins with the elimination of hydrogen chloride (HCl). The addition of thermal stabilizers (see also thermostability (biology) is necessary), at the same time they improve the weathering and aging resistance. If the PVC is exposed to elevated temperatures during further processing (for example through heated tool welding at 260 ° C), the additive package must be tailored to this For this purpose, compounds, for example stearates or carboxylates based on heavy metals such as lead , cadmium , tin , barium / zinc , calcium / zinc and calcium / aluminum / zinc such as cadmium stearate or lead stearate , are used (the metals catch in the melting process as "acid scavengers." "Release chlorine and form metal chlorides). Cadmium compounds as stabilizers were banned by the EU in 2001, and lead stabilizers are also to be replaced by 2015 (according to a source from 2010) (voluntary reduction target). Such metal-containing thermal stabilizers can be replaced by hydrotalcite (a magnesium substance). Aluminum hydroxycarbonate).
In addition, soft PVC can also contain antioxidants, heat stabilizers (support the shaping) such as organotin stabilizers and flame retardants (for example antimony trioxide ) as additives.
Phthalates , bisphenol A and the organotin compounds contained in PVC are considered to be endocrine (hormonally) effective. Such substances influence the reproduction and survival rate of amphibians and even the reputation behavior of frogs "so specifically that all substances are classified according to their mechanisms of action and environmentally relevant Concentrations could be detected. "
The additives mostly used (for pond liners ) (phthalates, bisphenol A, organotin compounds, etc.) are only slightly soluble in water, but low concentrations arise in the water depending on their solubility . They are partially extracted again from the water by being adsorbed on contact with biogenic solids and then disposed of together with the pond sludge. Sucked off soil sludge is usually used as mulch for surface composting . The compost can then contain higher levels of plasticizers and other additives (bisphenol A, tributyltin ) than the water. The additives are out of the water extracts , the water can be used as solvent and extractant resume additives and further dissolved out of the film. This creates a (physical) equilibrium .
Plasticizers
The addition of plasticizers gives the polymer plastic properties, such as flexibility and softness. Phthalic acid esters are mainly used as plasticizers . Chlorinated paraffins , adipic acid esters and phosphoric acid esters are of less importance . During thermoplastic processing, the plasticizers are embedded between the molecular chains of the PVC and thereby loosen the structure. The non-chemically bound plasticizers, which can make up "up to over 50% of the total mass", can be removed from a film, which also causes the films to become brittle after about 10 years. Broken PVC film in old film ponds and swimming pools is an indicator that the plasticizers originally contained have entered the environment through elution (leaching) or migration (further migration into other plastics, solids or microorganisms) or evaporation.
Plasticizers and additives can also migrate (in the case of foil ponds) from PVC into the plastics of the underlay fleece . The migration has a clear effect on swimming pools which (for better thermal insulation ) are made up of concrete-filled molded pieces of expanded polystyrene.In such cases, the film suppliers require that a geotextile fleece is inserted between the PVC film and polystyrene (otherwise guarantee claims will expire) .
This storage is a physical expansion of the structure, so that migration and gas release occur despite the low volatility. Depending on the intended use, this results in a sorbed surface layer or migration of the plasticizer into adjacent materials or through the air space into adjacent substances. Products on a different basis, which migrate more slowly due to significantly lower vapor pressures, are significantly more expensive, but are increasingly being used in Europe. These include, for example, acetyl tributyl citrate and 1,2-cyclohexanedicarboxylic acid diisononyl ester . Powder mixtures made of PVC with incorporated plasticizers and additives are called dry blends .
| Plasticizers (examples) | |
| Phthalic acid ester | Other carboxylic acid esters |
| Bis (2-ethylhexyl) phthalate (DEHP) | 1,2-Cyclohexanedicarboxylic acid diisononyl ester (DINCH) |
| Diisononyl phthalate (DINP) | Acetyl tributyl citrate (TBAC) |
When used as a pond liner, EPDM foil is used as an alternative , which is completely plasticizer-free and nevertheless easily stretchable, cold-tolerant and resistant (for further advantages see EPDM sealing membrane # Properties and use ). Their disadvantages are, for example, that they are only in black (with filler carbon black is produced) or white (in English-speaking countries as "EPDM Rubber white") and the joining technique of the vulcanization for lay is not so simple, such as the hot gas welding of PVC.
properties
PVC can be colored well and hardly absorbs water. It is resistant to some acids and alkalis and to a limited extent resistant to ethanol , oil and petrol . PVC is attacked by acetone , diethyl ether , tetrahydrofuran (THF), benzene , chloroform and concentrated hydrochloric acid . Hard PVC can be machined well, soft PVC poorly. It can be deformed without cutting at temperatures of 120 to 150 ° C. Connections can be made with adhesives (solvent adhesives, two-component adhesives) or plastic welding (various manual and machine welding processes). Solvent adhesives are mostly based on THF , to which 10 to 20% PVC powder (possibly post-chlorinated PVC) is added to increase the viscosity . A number of adhesives are available for ordinary rigid PVC (PVC-U), while only a few special adhesives such as Tangit PVC-C and Griffon HT 120 are offered for PVC-C. It is often recommended to clean the surfaces to be bonded beforehand with an appropriate solvent-based cleaning agent.
PVC burns with a yellow, heavily sooting flame and goes out quickly without any additional external flame. Due to its high chlorine content, PVC is, unlike other engineering plastics such as polyethylene or polypropylene , flame retardant. However, when PVC plastics fire, hydrogen chloride, dioxins and aromatics are produced.
PVC is a good insulator. The formation of dipoles and their constant realignment in electrical AC - field results in comparison to most other insulators to high loss tangents. Due to the high strength of the cable jacket and the good insulating properties, PVC low-voltage cables are very well suited for laying under plaster or outdoors.
| property | Hard PVC (PVC-U) | Soft PVC (PVC-P) | Chlorinated PVC (PVC-C) |
|---|---|---|---|
| Density in g / cm³ | 1.38 ... 1.40 | 1.20 ... 1.35 | 1.51 ... 1.64 |
| Thermal expansion coefficient in 10 −6 K −1 | k. A. | k. A. | 60 ... 70 |
| Melting point | Decomposition above +180 ° C | Decomposition above +180 ° C | |
| Glass temperature | +79 ° C | k. A. | |
| Impact strength in kJ / m² (according to DIN 53453) | low,> 20 | O. | k. A. |
| Notched impact strength in kJ / m² (according to DIN 53453) | 2 ... 75 | o. Br. | 12 |
|
Modulus of elasticity in MPa tensile modulus of elasticity (according to DIN 53457) |
1000 ... 3500 | k. A. | 2800 |
|
Modulus of elasticity in MPa, flexural modulus at 23 ° C |
k. A. | k. A. | 2800 |
| Water absorption in 24 hours | low | low | 0.04% |
| solubility | practically insoluble in water, soluble in organic solvents (acetone as well as esters and spot cleaners ), if molecular weight ≤30 kDa |
like PVC-U | similar to PVC-U |
| Chemical resistance | resistant to concentrated and diluted alkalis, oils, aliph. Hydrocarbons , decomposed by oxidizing mineral acids |
like PVC-U | resistant to concentrated and dilute acids, alkalis, oils, aliph. Hydrocarbons , not resistant to esters, ketones, chlorinated hydrocarbons , strong oxidizing agents. |
| Thermal conductivity in W / (m K) | low | low | 0.15 |
| Tensile strength in N / mm² (according to DIN 53455) | 50 ... 75 | 10 ... 25 | k. A. |
|
Elongation at Break / tear strength (according to DIN 53455) |
10 ... 50% | 170 ... 400% | k. A. |
| Yield stress in MPa at 23 ° C | k. A. | k. A. | 55 ... 60 |
|
Ball indentation hardness in MPa (10-second value according to DIN 53456) |
75… 155 | k. A. | 110 |
|
specific volume resistance (according to DIN 5348) |
> 10 15 Ω | > 10 11 Ω | k. A. |
| Surface resistance (according to DIN 53482) | 10 13 Ω | 10 11 Ω | 10 13 Ω |
| Use temperature | −50 ° C to +60 ° C | k. A. | up to +80 ° C… + 93 ° C, briefly +100 ° C |
|
Dielectric constant (according to DIN 53483) at 50 Hz at 1 MHz
|
3.5 3.0 |
4… 8 4… 4.5 |
k. A. k. A. |
| Tracking resistance (CTI) | 600 | k. A. | k. A. |
use
The advantage of PVC is its durability. Sunlight does not decompose it (provided that enough UV stabilizers are incorporated), the mechanical properties are not impaired. Water (including salty sea water ) and air have little or no damage to PVC. That is why PVC is mainly used for durable products. The products can be produced in a wide variety of colors and decorations.
PVC is very inexpensive because the raw material chlorine is an " inevitable by-product " in the production of caustic soda , which in turn is one of the most frequently used laboratory and industrial chemicals and is required in the chemical industry for so many processes (see caustic soda # Use ) that the demand cannot be met. Chlorine is therefore produced in excess and (since the decline of chlorinated hydrocarbons as a solvent) it has become a cheap waste product that can be gotten rid of. Among other things, the low price led to the boom in PVC products.
Solid PVC is used, for example, in garden and camping furniture and window frames . PVC pipes are less clogged due to the smooth inner surface, window profiles are easy to care for, require little maintenance and are weather-resistant,
PVC films have various uses, e.g. B. for water cores of water beds, as artificial leather or for foil sheets / bags in postage stamp albums, as pond foils and roofing membranes in the building sector and for floor coverings . Credit cards and mostly consist of PVC.
PVC is often used as a flame-retardant cable sheathing (insulation material for electrical cables), as an electrical switch box and as a pull-in tube for cables.
Foamed PVC in sheet form is used as a carrier material for advertising media, such as plotted lettering, images and graphics, mainly because of its low weight and easy processing. Special preparations are used in artistic installations and events. Heavily plasticized PVC films are offered as non-slip pads. Rigid PVC foam is used as a sandwich material in fiber composite technology . Areas of application are sport boats and wagon construction.
PVC is also used in pyrotechnics; more precisely, mostly as a so-called 'chlorine donor' . Due to the molecular release of Cl ions, the color effect of a pyrotechnic composition is intensified - usually with blue mixtures. PVC is also sometimes used as a binder in pyrotechnics.
In some areas of application, other plastics such as polypropylene (PP) and polyethylene (PE) are also used, with the advantage that there are no harmful substances that evaporate from soft PVC (typical plastic smell). The acid, oil and sea water resistance attributed to PVC is often not required. Some environmental associations recommend limiting the use of PVC to a few special applications.
economy
Windows with PVC frames are mainly exported. PVC is often used for pipes in cable trays and for membrane roofs, also for floor coverings. In 2001, 150,000 employees in 5,000 companies in Germany generated sales of 20 billion euros, around a quarter of the entire plastics industry.
Environmental aspects, disposal and recycling
Landfill
By 1989, about 70 percent of the waste was deposited. Rigid PVC does not decompose and does not damage water or air, but precisely because of this it takes up a lot of space on the garbage dump. Furthermore, no prognosis can be made as to whether the rigid PVC will eventually be attacked by microorganisms or chemical processes. However, it can be assumed with great certainty that the ingredients in soft PVC pollute the seepage water and thus the environment due to their plasticizer content. The landfilling of municipal waste with calorific value is no longer permitted in several European countries such as Germany, Austria and Switzerland.
Energy recovery
Energy can be obtained from the combustion process. The calorific value of 26.9 MJ / kg is relatively small compared to other plastics such as polypropylene (PP) of 52.6 MJ / kg. If PVC is burned, corrosive, gaseous hydrogen chloride is formed . In waste incineration plants, for example, this is neutralized with lime in the ventilation systems. The resulting residues are classified as hazardous waste .
Stabilizers containing heavy metals, such as lead distearate, pose a risk . For this reason, elaborate filter techniques are used in waste incineration plants to filter the harmful emissions. This means that the generation of energy is offset by high expenditure on ecological protection.
recycling
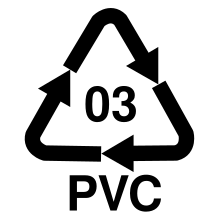
The recycling code for polyvinyl chloride is 03. When it comes to recycling, a distinction is made between a material and a raw material recycling method. There is a take-back system for PVC; Above all, floor coverings, roofing membranes, window profiles, electrical cables and PVC pipes are collected. The establishment and expansion of recycling structures is based on a voluntary commitment by the PVC industry (VinylPlus).
Material recycling
Thermoplastics, once formed into a workpiece, can be melted down again and shaped into a new product. The sequence of heat treatments, however, leads to a progressive loss of quality of the material (downcycling). An example of such an inferior end product is the beacon base (the holder into which red and white road barriers are inserted). Material recycling is therefore currently used almost exclusively where large quantities of a single-origin material are available.
The biggest problem with reprocessing is contamination. Cable waste from which the copper has been removed is still very dirty and must be cleaned in order to get back into a real cycle and to achieve the quality of a new material.
With the Vinyloop process , the PVC molecules and plasticizers can be extracted from PVC-containing composite materials with the solvent methyl ethyl ketone . After precipitation and drying, the mixture of polymers and plasticizers can be used to manufacture any PVC product. In Europe, there was only one plant in Ferrara (Italy), which was shut down in 2018.
Recycling of raw materials
Through pyrolysis , plastics can be broken down into substances that can be used for petrochemical purposes, such as methanol or synthesis gas. Naturally, these processes are primarily used for the recycling of mixed plastics, which can only be separated with great effort.
Health hazards
When the first workers in PVC production suffered from deformations of the extremities of the fingers or showed severe liver damage or liver cancer (hemangioendothelial sarcoma), occupational safety was improved in the production and processing of PVC. The "VC disease" has been recognized by the professional associations as an occupational disease . The basic material for PVC, vinyl chloride , can cause cancer in humans and has a mutagenic effect. Other raw materials used in PVC manufacture are also questionable. The maximum workplace concentration for PVC in the air is 0.3 mg / m³. In Switzerland, however, the value is 3 mg / m 3 (measured as respirable dust ).
When burned
When plastics containing chlorine such as PVC are burned in the presence of metal and carbon (e.g. in the presence of wood or dust), the poisonous gas phosgene can be formed.
Due to the plasticizers it contains
Soft PVC is physiologically questionable due to the plasticizers it contains, depending on the area of application. The use of soft PVC is problematic for toys, although it is widespread because of its low price and properties. Despite the low vapor pressure, plasticizers can get into the child's body through saliva, skin contact or the respiratory tract. The phthalate plasticizers are liver-part and nephrotoxic and are suspected to be carcinogenic. This was shown by several examinations in which clear traces were found in the blood. Diethylhexyl phthalate (DEHP) was classified by an EU working group in 2000 as harmful to fertility. Soft PVC with phthalate plasticizers was banned in the EU in 1999 for toys for small children.
“The human organism absorbs PVC plasticizers in higher quantities than previously assumed. Children are particularly at risk. The widespread plasticizers phthalates are considered to be extremely hazardous to health because they interfere with the human hormonal balance and damage reproduction or development "
Soft PVC is problematic in food packaging if it is not prevented by barrier layers from migrating into the food. Soft PVC should be avoided for fatty foods, as plasticizers are easily absorbed by the fat.
Elution, migration and sorption of plasticizers
With the "migration" of plasticizers and other additives, migration into other substances (liquids, neighboring plastic films and nonwovens), sorption (absorption of substances into the polymer) and permeation (transport of a substance through the polymer) must be taken into account . According to Fick's law of diffusion , an equilibrium is established during diffusion from or into the polymer. For example, there are interactions between the drug and the infusion tube , the drug only accumulates in the PVC before it reaches the patient in the desired dose. Styrofoam in thermally insulated walls of a swimming pool can, in contact with PVC film, remove plasticizers from the PVC, which can lead to embrittlement and premature breakage of the film.
While processing
One advantage of the PVC pond liners is the easy weldability of (new) PVC films, the joining of individual webs by hot gas welding can also lay and unskilled laborer will learn quickly. The fact that hazardous chlorine vapors or dioxins , benzene , naphthalene , phosgene , toluene or xylene and phthalates escape (when working outdoors) are often ignored . In a study on exposure to hydrogen chloride and phthalate during hot gas welding of PVC foils on 72 construction sites outdoors, no occupational exposure limit values were found, but such work in closed rooms (in other studies) did.
determination
In a fire test, the gases smell of hydrogen chloride. When burning on copper, the flame turns green (see Beilstein sample ). Both processes produce organochlorine compounds that are harmful to health. Therefore, only small amounts should be used for a firing test or a Beilstein test (outside of the test laboratories).
See also
literature
- Schrader, Franke: Small knowledge store plastic. Central Institute for Welding Technology Halle (ZIS). Technical-scientific treatise. Vol. 61. VEB German publishing house for basic industry, Leipzig 1970.
- Charles Levinson: PVC for example. Cancer in plastic production . Rowohlt, Reinbek 1985, ISBN 3-499-11874-2 .
- Robert Hohenadel, Torsten Rehm, Oliver Mieden: Polyvinyl chloride (PVC). Kunststoffe 10/2005, pp. 38-43 (2005), ISSN 0023-5563
- Andrea Westermann: Plastic and Political Culture in West Germany . Chronos, Zurich 2007, ISBN 978-3-0340-0849-5 , doi: 10.3929 / ethz-a-005303277 .
- Horst Pohle: PVC and the environment: an inventory. Springer-Verlag 1997. ISBN 978-3-642-59083-2
Web links
- Commitment by the European PVC industry
- Polyvinyl chloride without plasticizers - extensive material information and images on Materialarchiv.ch
Individual evidence
- ↑ a b c d e f g Entry on polyvinyl chloride in the GESTIS substance database of the IFA , accessed on July 30, 2017(JavaScript required) .
- ↑ Martin Bonnet: Kunststoffe in der Ingenieurapaltung , Vieweg + Teubner, Wiesbaden, 2009, p. 121.
- ^ Wolfgang Kaiser: Kunststoffchemie für Ingenieure , 3rd edition, Carl Hanser, Munich, 2011, p. 276 ff.
- ^ A b c Peter Elsner: DOMININGHAUS - plastics. Springer-Verlag, 2013, ISBN 978-3-642-16173-5 , p. 283 ( limited preview in Google book search).
- ↑ a b Additives and fillers in plastics (sic!) , Website about plastics technology, last accessed in February 2020.
- ↑ General report on treatment and recovery routes for PVC waste ; Federal Ministry of Agriculture, Forestry, Environment and Water Management, Vienna, December, 2002 (PDF file) , last accessed in February 2020.
- ↑ a b Hans Jürgen Wernicke and Joachim Großmann: “Environmentally friendly stabilization of PVC by synthetic hydrotalcites” ; Current newsreel of the GDCh; 2008, last accessed February 2020
- ^ Vinyl 2010. Voluntary commitment by the PVC industry. The European Council of Vinyl Manufacturers (industry association) (PDF file) , last accessed in February 2020.
- ↑ a b TBT - Organotin Compounds - A Scientific Inventory. Berlin, 2003, Federal Environment Agency Berlin, (PDF file) .
- ^ André Leisewitz, Hermann Kruse, Engelbert Schramm: Development of assessment bases for the substitution of environmentally relevant flame retardants ; Research report 204 08 542 (old) 297 44 542 (new), Environmental Research Plan of the Federal Minister for the Environment, Nature Conservation and Nuclear Safety, December, 2000 (PDF file) , last accessed in February 2020.
- ↑ a b c d e f P.Pfluger, B. Wasserrab, E. O'Brien, A.Prietz, P. Spengler, C.Schneider, A. Heussner, T.Schmid, B.Knörzer, JWMetzger, DRDietrich: Development and validation of in vitro test systems for the detection of endocrine disrupting foreign substances: chemical-analytical examination and biological proof of potentially endocrine disrupting substances in sewage treatment plant outlets or receiving waters ; Environment and its safety as a basis for life (BWPLUS); at pudi.lubw.de (State Agency for the Environment Baden-Württemberg); (PDF file) ;
- ↑ W.Kloas, C.Bögi, A.Gaete, O.Jagnytsch, A.Krüger, G.Levy, C.Lorenz, N.Neumann, R.Opitz, C.Pietsch, W.Schumacher, A.Tillack, A .Trubiroha, R.Urbatzka, C.Van Ballegooy, C.Wiedemann, S.Würtz, I.Lutz: Test procedure in amphibians for the detection of endocrine disruptors (ED) with effects on reproduction and the thyroid system ; in: Umweltbundesamt (editor): Proceedings 3rd status seminar Chemicals in the environment with an effect on the endocrine system. Scientific foundations of evaluation and regulation, Berlin, 2005; ISBN 3-8167-6968-3 , page 38; (PDF file) .
- ↑ Frauke Hoffmann, Werner Kloas: Suitability of the reputation behavior of the clawed frog as an end point for recording the effects of hormonally acting substances on aquatic ecosystems ; Environmental research plan of the Federal Ministry for the Environment, Nature Conservation, Building and Nuclear Safety; On behalf of the Federal Environment Agency; April, 2016, (PDF file) .
- ↑ Solubility: Entry on 4,4′-isopropylidenediphenol in the GESTIS substance database of the IFA , accessed on January 17, 2020(JavaScript required) .
- ↑ Peter Grathwohl: Sorption and desorption of hydrophobic organic pollutants in aquifer material and sediments ; in Jörg Matschullat, Heinz Jürgen Tobschall, Hans-Jürgen Voigt, et.al .: Geochemistry and Environment. Relevant processes in the atmosphere, pedosphere and hydrosphere
- ^ Robert Sattelberger, Marianne Heinrich, Gundi Lorbeer, Sigrid Scharf: Organotin compounds in the aquatic environment ; Publication of the Federal Environment Agency Vienna, 2002, ISBN 3-85457-661-7 ; (PDF file) .
- ↑ Lassen, Carsten et al. (2014): Survey of short-chain and medium-chain chlorinated paraffins , Copenhagen: The Danish Environmental Protection Agency, pp. 51, 55. ISBN 978-87-93283-19-0 .
- ↑ a b PHTALATE, the useful plasticizer with undesirable properties (PDF file) ; (German) Federal Environment Agency for Man and Nature.
- ↑ Ansilla Frank, Marc garlic, Benjamin Sandoz; Technology study for processing polyvinyl chloride (PVC) ; Fraunhofer Institute for Chemical Technology; Pfinztal; 2005; Page 4 (PDF file) .
- ↑ Gerd habenicht: Gluing. Springer-Verlag, 2013, ISBN 978-3-662-08085-6 , p. 611 ( limited preview in Google book search).
- ↑ a b c d e f g Technical information ( Memento from March 3, 2016 in the Internet Archive ) for Akatherm FIP products, at Kwerky.de
- ↑ Trovidur ( Memento of 3 March 2016 Internet Archive ) Röchling SE Co. KG
- ↑ a b c d e f g h Material properties of the Corzan pipes, retrieved from PVC-Welt.de, February 2016
- ^ Adolf Franck, Karlheinz Biederbick Kunststoff-Kompendium 1988, p. 264 ISBN 3-8023-0135-8 .
- ↑ a b c d e f g Wissenschaft-Online-Lexika: Entry on polyvinyl chloride in the Lexikon der Chemie , accessed March 6, 2008
- ↑ a b PVC (PolyVinyl Chloride) & CPVC (Chlorinated PolyVinyl Chloride) Specifications
- ↑ data sheet | CORE. Retrieved May 31, 2017 .
- ↑ a b Manfred Baerns, Arno Behr, Axel Brehm, Jürgen Gmehling, Kai-Olaf Hinrichsen, Hanns Hofmann, Ulfert Onken, Regina Palkovits, Albert Renken: Technische Chemie . John Wiley & Sons, 2014, ISBN 978-3-527-67409-1 , pp. 629 ( limited preview in Google Book search). , last accessed in February 2020.
- ^ Josef K. Felixberger: Chemistry for Beginners. ISBN 3662528207 p. 574 ( limited preview in Google book search).
- ↑ NICO - chemistry - it's all in the mix | NICO Europe GmbH . In: NICO Europe GmbH . ( nico-europe.com [accessed November 17, 2018]).
- ↑ Oliver Türk, Material use of renewable raw materials: Basics - Materials - Applications , Springer-Verlag, 2013. Restricted preview
- ↑ Working Group PVC and Environment eV (AGPU): Commitment of the PVC industry
- ↑ On the controversial history of the social struggle with cancer cases in the PVC manufacturing industry, cf. Andrea Westermann: Plastic and Political Culture in West Germany . Cape. 4th
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values , accessed on November 2, 2015.
- ↑ Plastics: interaction processes are often not taken into account. Plasticizers with a migration background ; at Medizin-und-technik.industrie.de
- ↑ Warning in the assembly instructions for a swimming pool; (PDF file)
- ↑ a b Underestimated dangers in plastics processing. Vapors hazardous to health from plastic welding / cutting. ; Information sheet from the Unfallkasse Nordrhein-Westfalen; at unfallkasse-nrw.de (PDF file)
- ↑ a b c d e f g h i Description of exposure of the professional association for the construction industry: Hot gas welding of PVC outdoors (PDF file) , at bgbau.de, last accessed in February 2020