Radioiodine therapy
The radioiodine therapy (RIT, also radioiodine, RIT) is a nuclear medicine therapies for the treatment of thyroid autonomy , of Graves' disease , the thyroid enlargement and certain forms of thyroid cancer . The radioactive iodine - isotope iodine -131 is used, which is a predominantly beta emitter with a half-life of eight days and is only stored in thyroid cells in the human body .
Radioiodine therapy is subject to special legal requirements in many countries and can only be carried out in Germany in an inpatient setting. The form of therapy has been used since the 1940s and is considered to have few side effects and is safe even in long-term follow-up. In Germany (as of 2014) there are around 120 therapy facilities in which around 50,000 treatments are carried out annually.
Areas of application and alternatives
The most common indications for radioiodine therapy are the autonomic dysfunction of the thyroid gland ( autonomous adenoma , multifocal autonomy, disseminated autonomy), Graves disease (Graves disease) and those forms of thyroid cancer (thyroid carcinoma) that store iodine: the papillary and follicular Thyroid cancer. Enlargement of the thyroid gland ( goiter ) without dysfunction is also increasingly being treated with radioiodine therapy.
Pregnancy is an absolute contraindication to radioiodine therapy in benign thyroid diseases. For general radiobiological considerations, it is recommended to avoid pregnancy for up to 6 months after radioiodine therapy. If a pregnancy does occur during this time, this is not a sufficient reason for an abortion . However, it is recommended to seek genetic counseling . Regarding radioiodine therapy for thyroid cancer during pregnancy, the theoretical probability of the occurrence of genetic damage can be calculated, but such damage has not yet occurred in reality.
Alternative treatment methods for the dysfunction are various forms of thyroid surgery and, in the case of autonomic adenomas, in certain cases sclerotherapy with alcohol . In some of the cases, Graves' disease can temporarily or permanently decrease in its activity ( remission ) during drug treatment with antithyroid drugs . As a final ("definitive") therapy, in addition to radioiodine therapy, surgery is also possible. In the case of thyroid carcinomas, radioiodine therapy can only be dispensed with after the operation in papillary carcinoma in the early stage pT1a . Drug therapy , surgery, and radioiodine therapy are used to treat benign thyroid enlargement .
When choosing between surgery and radioiodine therapy, the following arguments speak in favor of the non-surgical method: If the thyroid gland has already been operated on or already has (unilateral) paralysis of the vocal cord nerve ( recurrent palsy ), in older patients or with severe concomitant diseases, if the thyroid gland is relatively is small or the patient suffers from fear of surgery. Adolescent age is no longer considered a contraindication . The following arguments, however, speak for the operation: suspicion of malignancy ( malignancy ), by iodine induced hyperthyroidism ( hyperthyroidism ), pregnancy and lactation , florid (ocular involvement in Graves' disease endocrine ophthalmopathy ), signs of a narrowing (compression) of the neighboring structures ( trachea : stridor , Esophagus : pronounced swallowing disorder , neck vessels: upper congestion ), fear of radiation or large, cold areas of the thyroid gland.
Veterinary medicine
In veterinary medicine, radioiodine therapy is the therapy method of choice for hyperthyroidism in cats , but is only carried out to a limited extent due to the technical requirements and radiation protection requirements.
Legal requirements
In most countries, the implementation of radioiodine therapy is subject to certain statutory and quasi-statutory regulations. In many countries, radioiodine therapy can be carried out on an outpatient basis (i.e. without an inpatient hospital stay).
In Germany, the Radiation Protection Ordinance and the "Radiation Protection in Medicine" guideline issued by the Länder Committee for Nuclear Energy regulate the implementation of radioiodine therapy. They stipulate that the therapy may only be carried out on a nuclear medicine therapy station. The physician carrying out the work must have a corresponding handling permit , the issue of which is linked, among other things, to the " technical and expert knowledge in the field of the use of radioactive substances in diagnostics and therapy". The presence of a medical physics expert is necessary. The requirements of radiation protection must be taken into account with regard to structural and personnel aspects. In particular, a suitable decay plant for radioactively contaminated wastewater must be available. The supervising staff must be regularly instructed in radiation protection.
Outpatient therapies are possible in Austria . The border activities are stipulated by law. The presence of a medical physicist is also required in Austria.
In Switzerland , radioiodine therapy with an applied activity of up to 200 MBq may be carried out on an outpatient basis.
In the USA , radioiodine therapy can be performed on an outpatient basis up to an activity of 1110 MBq. The majority of therapies for benign thyroid diseases can be carried out on an outpatient basis.
Therapy principle and physical basics
The radioactive iodine isotope iodine-131 is available as sodium iodide in capsule form and in aqueous solution. As a rule, it is administered orally , but in exceptional cases (e.g. in the case of pronounced swallowing disorders) it can also be administered intravenously . When taken orally, the iodine is quickly absorbed through the gastric mucosa and released into the blood ( resorption ). The iodine reaches the thyroid cell via the sodium iodide symporter and is ultimately stored in the thyroid follicle. Under the action of TSH or TSH receptor autoantibodies , the uptake of iodine in the thyroid cells is increased. Autonomous parts of the thyroid take up iodine independently of TSH.
The special elegance of radio-iodine therapy lies in the fact that only thyroid cells absorb iodine - and thus radio-iodine as well - other organs do not store iodine. The iodine that is not stored in the thyroid gland is eliminated from the body within a short time via the kidneys and thus via the urine . Small amounts are excreted by the sweat glands and through the intestines. Iodine is excreted to a small extent by the salivary glands and the gastric mucosa , but is reabsorbed via the gastrointestinal tract. Because of these special properties of the iodine metabolism, it is possible to achieve a very high radiation dose in the target tissue ( focal dose ), while the other tissues only have a low radiation exposure .
Iodine-131 is a nuclide produced in nuclear reactors and has a half-life of 8.02 days. When it decays into stable xenon , a beta particle is released - with a maximum energy of 0.61 MeV and an average range in tissue of 0.5 mm. This radiation is responsible for the therapeutic effect. In addition, gamma radiation with the main peak at 364 keV is released, which can leave the thyroid gland and is therefore responsible on the one hand for the undesired radiation exposure of the patient and the environment, but on the other hand can also be used for imaging and the radioiodine test .
The beta rays cause damage to the DNA in the vicinity of the thyroid cells , in particular double-strand breaks, which ultimately lead to the initiation of programmed cell death ( apoptosis ) (→ mechanism of action of radiation therapy ).
Radioiodine therapy for benign thyroid diseases
preparation
Iodine leave
In order to achieve optimal absorption of iodine in the thyroid gland, additional sources of iodine should be avoided before the radioiodine test and therapy with radioactive iodine (iodine abstinence ). Such sources of iodine are, in particular, contrast media containing iodine , the antiarrhythmic amiodarone and certain disinfectants containing iodine . A waiting period of 6 weeks is sufficient for water-soluble contrast media. After using fat-soluble contrast media or the likewise fat-soluble amiodarone, the thyroid gland can be blocked for many months.
Significant amounts of iodine are found in many multivitamin products and dietary supplements , as well as in sea fish, seafood and certain products made from algae. These sources of iodine should be avoided for about a week before the iodine test and therapy. The ingestion of iodine from iodized table salt and industrially manufactured foods prepared with it is practically impossible to completely avoid.
Metabolic adjustment
If possible, radioiodine therapy should not be carried out if the metabolism is manifestly hyperthyroid , as the overactive function can be increased by the release of the thyroid hormones thyroxine (T 4 ) and triiodothyronine (T 3 ). Therefore, normal values for T 4 and T 3 should be set with the lowest possible dose of thyreostatics in the weeks prior to radioiodine therapy . However, anti-thyroid drugs reduce the uptake of iodine by the thyroid gland, so it is recommended that these be discontinued at least one to two days before radioiodine therapy.
In the case of thyroid autonomy , the TSH value should be suppressed as much as possible at the time of radioiodine therapy in order to minimize iodine uptake in non-autonomous parts of the thyroid via the thyrotropic control loop . If necessary, thyroid hormones must be given in the preparation phase. For euthyroid goiter, therapy is usually carried out without accompanying thyroid-directed medication.
Determination of the appropriate therapy activity
The German guideline "Radiation Protection in Medicine" stipulates: "When planning a nuclear medicine treatment, the dose for the organs or tissues to be treated [...] must be determined in advance and the activity to be administered afterwards. If patient-specific parameters are required, individual measurements and data are to be used. "
When determining the activity required for therapy in Germany and Austria, a medical physicist must be involved in addition to the nuclear medicine specialist. The target dose, the target volume and the integral of the activity in the thyroid gland over time are included in the calculation .
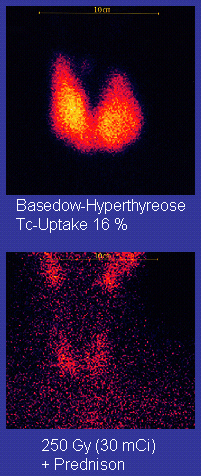
The target volume of radioiodine therapy and the energy dose aimed for in the target volume depend on the disease to be treated. With Graves disease, with thyroid enlargement without autonomy and with disseminated autonomy, the sonographically determined total volume of the thyroid is assumed as the target volume, with unifocal and multifocal autonomy only the volume of the adenomas. The target doses aimed for according to the guidelines of the German Society for Nuclear Medicine for radioiodine therapy in benign thyroid diseases are given in the following table.
| Target dose aimed for in radioiodine therapy | effective half-life | ||
|---|---|---|---|
| illness | targeted target dose | Euthyroidism | Hyperthyroidism |
| Unifocal autonomy | about 300 to 400 Gy | 4.8 days | 4.2 days |
| Multifocal autonomy | about 150 Gy | 5.5 days | 4.8 days |
| Disseminated autonomy | about 150 Gy | 5.5 days | 4.8 days |
| Graves disease | - | - | 4.2 days |
| Ablative concept | about 200 to 300 Gy | - | - |
| Function-optimized concept | about 150 Gy | - | - |
| Goiter to reduce the size | about 120 to 150 Gy | 5.5 days | - |
The activity-time integral can be determined with the radioiodine test. For this purpose, a small amount of radioiodine is administered (usually orally ): about 1 to 5 MBq iodine-131 or - less often - 5 to 10 MBq iodine-123. At certain times, the activity above the thyroid gland is measured and compared to the initial activity and its natural decay. The maximum absorption ( uptake ) in percent and the effective half-life in days are to be determined. The more measurement points there are, the more precisely the area under the curve can be determined. For reasons of practical (outpatient) feasibility, measurements are usually only taken after 24 and 48 hours, and possibly only after 24 hours. In Graves' disease, because of the accelerated iodine metabolism, an initial measurement is required after four to eight hours. To accurately determine the effective half-life, a further measurement is necessary after four to eight days. Because there are only minor differences in the effective half-life between the individual patients with the same disease and the same metabolic status, the effective half-life can also be assumed as an empirically determined value (see table).
The therapeutic activity to be applied can then be calculated using the Marinelli formula (after Leonidas D. Marinelli , 1906–1974, Argonne National Laboratory ).
Here, A is the activity to be calculated, HD is the targeted focus dose , V is the determined target volume, K is an empirically determined constant of 24.7, U max is the maximum uptake and t 0.5 eff is the effective half-life.
Cortisone pretreatment for endocrine orbitopathy
An endocrine ophthalmopathy (eo) in Graves' disease may get worse during radioiodine therapy or first appear. With pre-existing eO, treatment with glucocorticoids (cortisone) is recommended from the day of radioiodine therapy or the day before ; the dosage depends on the severity of the eye disease. In Graves' disease without endocrine orbitopathy, the prophylactic administration of glucocorticoids is controversial. The possible side effects and contraindications of cortisone therapy ( diabetes mellitus , stomach ulcers and electrolyte disorders ) must be observed.
enlightenment
Before starting the radio-iodine test and radio-iodine therapy, an explanation is given . In this, the patient is informed about the course of the test and therapy as well as the risks and side effects of radioiodine therapy . In countries in which radioiodine therapy can only be carried out on an inpatient basis, the subject of the educational discussion is that the patient must not leave the therapy ward after the therapy has been administered. The patient's attention is also drawn to the fact that regular follow-up examinations are medically necessary and required by law (see Follow-up Care section ).
execution
After the mostly peroral - in exceptional cases intravenous - administration of iodine-131, the patient must remain in the therapy ward in Germany for at least 48 hours. It is recommended to stay sober about an hour after oral intake until the radioiodine is largely absorbed.
Regular measurements of the radiation are then usually carried out; these allow conclusions to be drawn about the activity remaining in the patient's entire body or in the thyroid gland . The former is important for the time of discharge after therapy , the latter for the maximum value and the time course for monitoring the success of the therapy. The actually achieved maximum uptake (U max ) and the actually achieved effective half-life (t 0.5 eff ) can be determined and the actually achieved focus dose (HD) can be determined by changing the Marinelli formula (see above ). The target volume V and the empirically determined constant K are unchanged.
If the focal dose deviates significantly upwards from the planned value, there is a higher probability that an underactive thyroid will develop after the therapy and follow-up care should be planned accordingly.
If, on the other hand, the focus dose deviates significantly downwards, an additional dose of radioiodine can still be given during the inpatient treatment stay in order to still achieve the desired target dose and to ensure the success of the therapy. It should be noted that due to the early effect of radioiodine therapy (“stunning”), the uptake of the second dose is regularly lower than that of the first.
By administering small amounts of non-radioactive iodine or lithium after the administration of radioiodine, the remaining radioiodine in the thyroid and thus the effective half-life t 0.5 eff can be extended and a herd dose that is up to 30% higher can be achieved. Particularly with lithium, the risks and side effects as well as the narrow therapeutic range must be taken into account.
Risks and Side Effects
Radiation exposure
Due to the biological properties of iodine and the physical properties of iodine-131, radioiodine therapy only causes a low level of radiation exposure of the organs that do not absorb radioiodine. The radiation exposure is in the adjacent thyroid structures such as the larynx or the parathyroid low - due to the short range of the beta radiation from the thyroid gland. Some tissues that express the sodium iodide symporter , including the salivary glands , stomach and female breast , temporarily accumulate iodine. The kidneys , urinary bladder and intestines are involved in excreting the radioiodine that is not stored in the thyroid. In all of these tissues there is a low activity-time integral, the radiation exposure is correspondingly low. With an uptake of radioiodine in the thyroid gland of 25%, it is given as 0.46 mGy / MBq for the stomach wall , 0.04 mGy / MBq for the ovaries , 0.07 mGy / MBq for the red bone marrow , and 0.035 mGy for the liver / MBq and the testes with 0.028 mGy / MBq. In the thyroid target tissue, on the other hand, the (desired) radiation dose is four to five powers of ten higher: with an uptake of 25% at 350 mGy / MBq.
The cohort studies by Hall 1992 with over 45,000 patients and Ron 1998 with over 35,000 patients did not find any increased cancer mortality in patients treated with radioactive iodine. The American Thyroid Association advises of a possible, minimally increased incidence of thyroid cancer after treatment.
Acute side effects
Acute side effects only occur in a few cases ; these are mostly harmless. The most common is radiothyroiditis, a painful inflammatory reaction of the thyroid gland caused by the acute effects of radiation, which can occur about three to five days after ingestion of the radioiodine. Cooling and anti-inflammatory drugs such as non-steroidal anti-inflammatory drugs or glucocorticoids can usually relieve the symptoms, which subside within a few days. A swelling accompanying the inflammation of the thyroid gland can only lead to serious problems such as shortness of breath if the windpipe is narrowed (tracheal stenosis), so preventive anti-inflammatory treatment is recommended in these cases.
Inflammatory swellings of the salivary glands usually only occur during higher therapy activities.
Pre-existing hyperthyroidism can worsen about seven to ten days after the start of treatment, due to the onset of cell breakdown and the associated release of hormones that were stored in the thyroid. This complication can be avoided by establishing a normal metabolic situation prior to treatment.
A rare side effect after radioiodine therapy due to thyroid autonomy ( e.g. autonomic adenoma ) is the additional occurrence of immunogenic hyperthyroidism a few weeks later. This phenomenon is called Marine-Lenhart Syndrome and is mostly self-limiting. The frequency is given as 0.5 to 1%. In some of the cases, however, a second radioiodine therapy must be carried out because of the newly developed hyperthyroidism.
Discharge after therapy
In Germany, the minimum length of stay on the therapy ward is 48 hours. The discharge depends on the residual activity remaining in the body. In 1999, the limit for residual activity was increased: the dose rate must not exceed 3.5 µSv per hour at a distance of 2 meters from the patient , which means that a radiation exposure of 1 mSv is not exceeded within a year at a distance of 2 meters. This corresponds to a residual activity of around 250 MBq . Similar regulations apply to Austria.
In Switzerland, radiation exposure of a maximum of 1 mSv per year for “other people” and a maximum of 5 mSv per year for relatives of the patient (“non-professional caregivers”) may not be exceeded. In the case of discharge after radioiodine therapy, a dose rate of at most 5 µSv per hour at a distance of 1 meter is therefore permissible, which corresponds to a residual activity of around 150 MBq.
In Germany, the average length of stay is around three to five days and depends largely on the target volume. In the case of very large currents , the length of stay can reach ten days.
Upon discharge, the patient is informed of any radiation protection measures that may still need to be observed. These particularly concern the handling of young children and pregnant women. The patient must be informed of possible problems with radioactivity measurements - at airports, nuclear power plants, waste disposal - and the patient will be given a corresponding certificate if necessary.
In special cases - for example an acute illness of the patient, which necessitates an examination and treatment of the patient outside the therapy ward - an early discharge is possible in Germany. This must be reported to the supervisory authority up to a dose rate of 17.5 µSv / h; approval must be obtained from 17.5 µSv / h. If the patient is transferred to another ward, the responsible radiation protection officer must ensure that suitable radiation protection measures are taken there, for example a temporary control area is set up.
Follow-up care and successes
In Germany, the doctor who performed the radioiodine therapy is also responsible for the follow-up care. The competent doctor responsible for carrying out the treatment must record and document the effects and side effects of the nuclear medicine treatment by means of suitable follow-up examinations carried out at appropriate intervals; if necessary, he has to initiate treatment. The nuclear medicine doctor can hand over parts of the follow-up care to a professionally qualified doctor, who has to inform him of the results of the follow-up care. However, this does not relieve the nuclear medicine doctor from his responsibility for aftercare.
Depending on the thyroid disease being treated and the metabolic status or the pre-treatment with medication, the first checks of the thyroid laboratory values are carried out after four to six weeks, in the case of Graves' disease, possibly earlier, in order to detect the development of an underactive thyroid in good time. In particular with pre-existing endocrine orbitopathy, hypofunction can have unfavorable effects and must therefore be treated early.
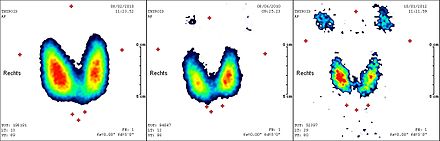
In most cases, the full effect of the radioiodine therapy occurs in the first three to six months, so that after this period a final examination is required to assess the therapeutic effect and success. In addition to the determination of thyroid hormones , this usually also includes a thyroid sonogram and a scintigram . Since in some of the cases a certain delayed effect of the radioiodine therapy can be expected even after six months, the indication for a repetition of the treatment should not be made too early.
Different therapy goals and success rates apply to the various indications for radioiodine therapy. For the autonomy of the thyroid gland, the goal is to switch off the autonomous parts of the thyroid gland. With a target dose of 300 to 400 Gy, a success rate of over 90% is given. About 10% of the patients have to be treated permanently with thyroid hormones because of an underactive thyroid after therapy ("substitution"). In the case of focal autonomy, a decrease in the volume of the autonomous areas by around 80% can be expected. The decrease in the volume of the total thyroid gland is 20 to 50%.
In Graves' disease, the goal is to permanently eliminate the hyperfunction. With the ablative therapy concept with the target dose of 200 to 300 Gray, the success rate is over 90%. However, 80 to 90% of patients are then permanently dependent on substitution with thyroid hormones. In the so-called function-optimized concept with a targeted focus dose of around 150 Gray, the rate of sub-functions requiring treatment is only 40%, but the success rate is only around 70%. This low success rate is unacceptable, particularly in the case of difficult courses with overfunction that is difficult to control with drugs, eye involvement ( endocrine orbitopathy ) or repeated relapses, so that the ablative therapy concept is now predominantly used. For the alternative treatment method to radio-iodine therapy, goiter resection , an underfunction requiring treatment is also to be expected.
The goal of therapy for goitre without hyperfunction is primarily a reduction in the size of the thyroid gland and the elimination of any symptoms caused by the size of the goiter. With an initial volume of 50 to 100 ml, a size reduction of around half is to be expected, with very large goiter with a volume of over 250 ml only a reduction of around 30 to 40%. In a total of over 80% of the cases an improvement of the symptoms is achieved.
Radioiodine therapy for malignant thyroid diseases
Basics
The epithelial carcinomas of the thyroid gland, namely the most common subtypes papillary carcinoma (about 70%) and follicular carcinoma (about 20%), and their settlements ( metastases ) still have marked similarities with their original tissue . On the one hand , they express the sodium iodide symporter and therefore have the ability to store iodine, on the other hand they produce thyroglobulin , which can therefore serve as a tumor marker . Thyroid carcinomas are often noticeable as cold nodules in a thyroid scintigraphy , i.e. the tendency to store iodine is less pronounced than in the surrounding healthy thyroid tissue. When stimulated with high levels of TSH , there is also an uptake of iodine by malignant ( malignant ) thyroid cells.
application
A distinction must be made between radioiodine therapy for the removal ( ablation ) of any residual healthy thyroid tissue in the sense of a supportive therapy that simplifies aftercare ( adjuvant ) and the treatment of possibly existing tumor residues or settlements (metastases) with a claim to healing ( curative therapy ) or at least to extend life and alleviate symptoms ( palliative therapy ).
An indication of ablative radioiodine therapy exists in almost all cases of epithelial thyroid cancers. Only in the case of papillary microcarcinoma (size smaller than 1 cm, pT1 N0 M0 ) can an ablative radioiodine therapy be dispensed with.
There is usually no indication for radioiodine therapy in medullary and anaplastic thyroid carcinoma, since these tumors usually do not store iodine. Pregnancy and breastfeeding are considered an absolute contraindication.
preparation
surgery
Before radioiodine therapy for epithelial thyroid carcinoma, thyroid surgery, usually performed as a thyroidectomy , is always performed. A small residue of benign thyroid tissue almost always remains. In the case of difficult surgical conditions - for example in the case of very large goiter or previously operated patients - this remainder can also be larger, but this does not mean any disadvantage for the further treatment of the patient and their prospects for survival ( prognosis ). It is therefore not recommended to strive for an absolutely complete removal of the thyroid gland in order to avoid damage to the recurrent laryngeal nerve behind the thyroid gland .
If there is residual tumor, there are already settlements ( metastases ) or a local relapse ( relapse ), an attempt should first be made surgically to reduce the amount of malignant tissue as far as possible.
Iodine leave
Even more than with radio-iodine therapy for benign thyroid diseases (see above ) , care must be taken in the phase before radio-iodine therapy for thyroid carcinoma that the patient is not given any additional iodine.
Stimulation of the thyroid tissue and iodine test
Before the ablative radioiodine therapy, a (shortened) radioiodine test (see above ) lasting 24 hours should be carried out. In the case of a high uptake, the therapy is carried out with reduced activity in order to avoid local complications - and the possibility is accepted that further treatment will later be necessary to ablate any residues. If the uptake is very high, a second operation to reduce the thyroid tissue should be considered.
To increase the ablative therapy the uptake of radioiodine in the thyroid remnant, or reach for the curative or palliative therapy at all appreciable recording, the remaining thyroid cells have a high level of TSH stimulated ( stimulated ) are. The TSH value should be above 30 mU / l, normal (depending on the laboratory) are around 0.4 to 4 mU / l. This high value is usually achieved by initially not treating the patient with thyroid hormones after the operation (substitution), as is usually the case after a complete (thyroidectomy) or extensive thyroid removal (subtotal goiter resection ) in benign thyroid diseases. About three to four weeks after the operation, the patient is then in a deep underactive thyroid ( hypothyroidism ). Often there is an inability to work due to the subfunction and the patient should be advised that in this phase they are not allowed to drive a motor vehicle or control dangerous machines due to their reduced ability to react .
As an alternative to hypofunction, genetically engineered, recombinant , human TSH ( rhTSH ) has been available for some years now , which can be given intramuscularly and with which TSH levels are usually well above 30 on the third and fourth day by injections on two consecutive days mU / l.
Possible advantages of using rhTSH over hypothyroidism are: better general well-being; ability to work received; Lack of a sustained growth stimulus for cancer cells that may still be present due to increased TSH for several weeks; Lower concentration of iodine-131 in the serum due to normal kidney function - in hypothyroidism, kidney function is usually temporarily restricted - and thus lower radiation exposure. The success rates of ablative radioiodine therapy are equally high in hypofunction and with rhTSH.
The following possible disadvantages should be mentioned: significantly shortened effective half-life ; increased amounts of non-radioactive iodine in the patient's body due to continued administration of thyroid hormones (containing iodine); lack of evidence of equivalence in the treatment of metastases; Inability to follow up a diagnosis with radioiodine therapy, as the TSH level drops again within a few days; high costs.
execution
The standard activity for ablative radioiodine therapy is 3.7 GBq . (In the outdated unit, this corresponds to Curie 100 mCi .) If significant remnants of thyroid tissue remain after the operation and before the first (ablative) radioiodine therapy, a lower activity of 1.1 to 1.85 GBq (corresponding to 30 to 50 mCi) are used to avoid radiation-induced inflammation of neighboring tissues.
If - even after the surgical possibilities have been exhausted - it can still be assumed that malignant tissue has remained in the patient's body, i.e. a residual tumor, a local recurrence or metastases are present, higher activities are used. These are mostly in the range from 3.7 to 11.1 GBq (100 to 300 mCi), in individual cases significantly higher.
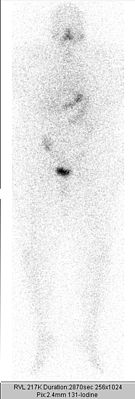
A full body scintigram is made a few days after therapy . The therapy is repeated at about three-month intervals until neither the scintigram, the ultrasound image of the neck region, nor the tumor marker thyroglobulin indicate any significant remaining thyroid tissue (benign or malignant). A total activity of up to 74 GBq (2000 mCi) over all radioiodine treatments carried out is usually tolerated without problems in otherwise healthy patients. When doing higher activities, there is an increased risk of permanent damage to the bone marrow as a blood-forming organ.
With regard to discharge after therapy, the same deadlines and limit values apply as for the treatment of benign thyroid diseases (see above ) .
Risks and Side Effects
In the case of the undesirable effects of radioiodine therapy, a distinction must be made between the short-term and acute side effects from the long-term and chronic side effects , and their respective frequencies and consequences (“ risks ”) must be observed.
Compared to the therapy of benign thyroid diseases, thyroid carcinoma sometimes involves significantly higher activities. With the desired higher radiation dose in the target tissue ( focal dose ), higher radiation doses in other tissues are also associated.
Loss of appetite, changes in taste sensation, nausea and irritation of the salivary glands occur relatively frequently during therapy; Neck pain or swelling, headache, or temporary changes in blood count are rarely found.
Serious long-term effects are rare and mostly affect the salivary and lacrimal glands (especially reduced tear or saliva production), bone marrow (very rare in patients> 45 years of age, bone marrow depression or myeloid leukemia), lungs (very rarely radiation-induced pneumonia, especially worsening in the case of pre-existing pneumonia, or new occurrence with diffuse lung metastases) and ovaries (temporary lack of ovulation, slightly increased rate of malformations in pregnancy within the first year after therapy) or testes ( azoospermia - rarely permanent). The benefits of radioiodine therapy exceed the rate of side effects by 4 to 40 times. The expected incidence of secondary cancers or leukemia is dose-dependent. A minimal increase in secondary carcinomas was found in the organs (salivary glands, large intestine and bladder), in which iodine-131 also temporarily accumulates in significant quantities during therapy. An English study on 7417 patients showed a statistically significant decrease in the incidence of cancer of the airways ( bronchi and trachea ).
The undesirable effects on the salivary glands can be reduced if the patient chews gum or sucks sour candy during the treatment. Both of these increase the flow of saliva, shorten the dwell time of the radioactive iodine in the salivary glands and thus reduce the radiation dose in the salivary glands. If there is an inflammatory reaction of the salivary glands caused by radiation ( sialadenitis ), the symptoms can be alleviated by local cooling and the use of non-steroidal anti-inflammatory drugs .
Follow-up care: radioiodine diagnostics
Radioactive iodine is used not only for treating thyroid cancer, but also for follow-up care after thyroid surgery and ablative radioiodine therapy.
The procedure - with iodine abstinence, stimulation of the TSH with subsequent determination of the tumor marker thyroglobulin and oral administration of iodine as well as carrying out the whole-body scintigraphy - corresponds to the procedure for ablative therapy. On the other hand, the level of activity administered differs, usually around 100 to 400 MBq iodine-131 or 40 to 200 MBq iodine-123.
An indication for radioiodine diagnostics is usually three to six months after ablative radioiodine therapy to control therapy, if the tumor marker thyroglobulin rises or if there is any other suspicion (e.g. on the basis of clinical findings or imaging tests ) of a relapse of the cancer ( relapse ). If and when for patients who because of their initial findings as a high-risk patients (high-risk) a Radiojoddiagnostik makes sense were classified, even without concrete suspicion of a recurrence, the subject is science. There are no guidelines or evidence-based results on this.
The detection of residual tissue or metastases in the context of radioiodine diagnostics usually leads to a new radioiodine therapy.
Successes and failures
The chances of a cure are generally very good in the case of differentiated thyroid carcinomas, especially since they are accessible to radioiodine therapy. For treated patients, mean 10- year survival rates of over 90% for the papillary and about 80% for the follicular variant are given. The prognostic factors include the patient's age and the size, extent and histological differentiation of the tumor; Lymph node metastases do not seem to have a major impact on the prognosis . Due to the increased risk of recurrence, consistent follow-up care is particularly important.
Studies with large numbers of patients and over long observation periods show that radioiodine therapy lowers the frequency of local relapses (local recurrences ) and the risk of dying from thyroid cancer ( lethality ), especially when performed routinely after surgery.
The results of radioiodine therapy in patients who already have distant metastases are inconsistent. Metastases in the liver or lungs that store iodine are easily treatable. In contrast, even bone metastases that absorb iodine well can hardly be influenced. Overall, radioiodine therapy is used for metastases when a cure is no longer possible through surgery. Before radioiodine therapy, however, an attempt should be made to surgically reduce the total tumor mass. If a (renewed) operation would possibly be associated with considerable local damage, in individual cases with very slowly growing tumors one will decide against the operation with subsequent radioiodine therapy, but only treat with L-thyroxine.
The situation is difficult if the metastases lose their ability to store iodine in the course of treatment (de- differentiation ). These metastases are not recognized in radioiodine diagnostics and are not reached by radioiodine therapy. Positron emission tomography (PET) with fluorodeoxyglucose (FDG) is used for diagnosis . Since chemotherapy and radiation therapy do not allow a cure either, an attempt is made to achieve a re-differentiation with the administration of isotretinoin . The subsequent radioiodine therapy shows at least partial success in about 20 to 35% of the cases.
history

In the run-up to the use of radioiodine therapy, postoperative postoperative irradiation (with X-rays) was generally used and considered effective in thyroid carcinoma. However, the diagnosis of thyroid carcinoma was still a major problem in the middle of the last century, so that there is likely to be an overlapping of terms in the literature of this time.
In 1939 and 1940 Joseph G. Hamilton published work on the iodine metabolism of the thyroid gland, using radioactive iodine in healthy subjects and those with goitre of various pathogenesis . In previous years he had already researched the effects of radioactivity on the human body.
In 1942, Saul Hertz of the Massachusetts General Hospital and the physicist Arthur Roberts published their report on the first radioiodine therapy (1941) for Graves' disease , at that time still predominantly with the isotope iodine-130 (half-life 12.4 hours). A few months later - also in 1941 - Joseph Hamilton and John H. Lawrence carried out the first therapy with iodine-131 - the isotope that is still used today. In 1942, AS Keston and co-workers described in a case study the use of radioactive iodine for therapeutic and diagnostic purposes in a metastasis of thyroid carcinoma in the thigh bone that had not responded to the then common therapy with X-rays. Another case study from 1942 shows that this was by no means a routine procedure for thyroid carcinoma.
Samuel M. Seidlin (1895–1955, then head of the endocrinological department of the Montefiore Hospital in New York City ) first used iodine-131 in metastatic thyroid cancer in 1946.
The first radio-iodine therapy for thyroid cancer in Europe was carried out in 1948 at the Luisen-Hospital Aachen by Cuno Winkler .
literature
- F. Grünwald, C. Menzel. Radioiodine therapy. In: T. Kuwert, F. Grünwald , U. Haberkorn , T. Krause: Nuclear medicine. Stuttgart / New York 2008, ISBN 978-3-13-118504-4 .
-
Medical guidelines :
- Guideline on radioiodine therapy (RIT) for benign thyroid diseases of the German Society for Nuclear Medicine (DGN) . In: AWMF online (as of 10/2015)
- Guideline procedural instruction for the radioiodine test. of the German Society for Nuclear Medicine (DGN). In: AWMF online (as of 10/2014)
- Guideline procedural instructions for radioiodine therapy (RIT) in differentiated thyroid cancer. of the German Society for Nuclear Medicine (DGN). In: AWMF online (as of 10/2015)
- Guideline Procedural instructions for iodine-131 full-body scintigraphy in differentiated thyroid carcinoma. of the German Society for Nuclear Medicine (DGN). In: AWMF online (as of 07/2017)
Web links
- L.-A. Hotze: The history of radioiodine therapy. ( Memento from June 24, 2010 in the Internet Archive )
Individual evidence
- ^ Frank Grünwald, Karl-Michael Derwahl: Diagnosis and therapy of thyroid diseases. Frankfurt / Berlin 2014, ISBN 978-3-86541-538-7 , p. 109.
- ↑ a b c d e f g h i j k l m n o p q F. Grünwald, C. Menzel: Radioiodine therapy. In: T. Kuwert, F. Grünwald , U. Haberkorn , T. Krause: Nuclear medicine. Stuttgart / New York 2008, ISBN 978-3-13-118504-4 .
- ↑ a b Lothar-Andreas Hotze, Petra-Maria Schumm-Draeger: thyroid diseases. Diagnosis and therapy. Berlin 2003, ISBN 3-88040-002-4 .
- ↑ a b Guideline of the German Society for Nuclear Medicine (DGN): Radioiodine Therapy (RIT) for benign thyroid diseases.
- ^ ME Peterson: Radioiodine treatment of hyperthyroidism . In: Clin. Tech. Small Anim. Pract. , 21 (1) / 2006, pp. 34-39, PMID 16584029 , ISSN 1096-2867
- ↑ a b Guideline "Radiation Protection in Medicine" (administrative regulations-im-internet.de); accessed on March 25, 2018.
- ↑ LD Marinelli, EH Quimby, GJ Hine: Dosage determination with radioactive isotopes; practical considerations in therapy and protection. In: The American journal of roentgenology and radium therapy . tape 59 , no. 2 , 1948, p. 260-281 , PMID 18905884 .
- ↑ Guideline of the German Society for Nuclear Medicine (DGN) : procedural instructions for the radioiodine test. ( awmf.org ).
- ↑ a b I. S. Robertson: Dosimetry of radionuclides. In: S. Falk (Ed.): Thyroid disease. Raven, New York 1997. Quoted from: F. Grünwald, C. Menzel. Radioiodine therapy. In: T. Kuwert, F. Grünwald , U. Haberkorn , T. Krause: Nuclear medicine. Stuttgart, New York 2008, ISBN 978-3-13-118504-4 .
- ^ P. Hall, G. Lundell, A. Mattsson, K. Wiklund, L. -E. Holm, M. Lidberg, JD Boice, G. Berg, G. Bjelkengren, U. -B. Ericsson, A. Hallquist, J. Tennvall: Leukemia incidence after iodine-131 exposure . In: The Lancet . tape 340 , no. 8810 , 1992, pp. 1-4 , doi : 10.1016 / 0140-6736 (92) 92421-B , PMID 1351599 .
- ↑ Elaine Ron, Michele Morin Doody, David V. Becker, A. Bertrand Brill, Rochelle E. Curtis, Marlene B. Goldman, Benjamin SH Harris III, Daniel A. Hoffman, William M. McConahey, Harry R. Maxon, Susan Preston -Martin, M. Ellen Warshauer, F. Lennie Wong, John D. Boice, for the Cooperative Thyrotoxicosis Therapy Follow-up Study Group: Cancer Mortality Following Treatment for Adult Hyperthyroidism . In: JAMA . tape 280 , no. 4 , 1998, pp. 347-355 , PMID 9686552 ( Cancer Mortality Following Treatment for Adult Hyperthyroidism ).
- ^ Radioactive Iodine. American Thyroid Association information , accessed October 5, 2012.
- ↑ Art. 37 of the ordinance on handling unsealed radioactive sources at admin.ch; Retrieved March 20, 2011.
- ↑ Appendix 5 of the Ordinance on Handling Unsealed Radioactive Sources at admin.ch; Retrieved March 20, 2011.
- ↑ a b c d Radioiodine therapy in differentiated thyroid carcinoma . Retrieved January 9, 2016.
- ↑ a b c S. M. Chow: Side effects of high-dose radioactive iodine for ablation or treatment of differentiated thyroid carcinoma . In: Hong Kong College Radiologist . No. 8 , 2005, p. 127–135 ( hkcr.org (PDF) [accessed March 13, 2008]).
- ↑ DJ Handelsman, AJ Conway, PE Donnelly, JR Turtle: Azoospermia after iodine-131 treatment for thyroid carcinoma. In: British Medical Journal. 281, No. 6254, 1980, p. 1527, PMC 1714914 (free full text).
- ↑ Guideline of the German Society for Nuclear Medicine (DGN) : procedural instructions for iodine-131 whole-body scintigraphy in differentiated thyroid carcinoma. ( online ).
- ↑ Austrian Society for Surgical Oncology: prognosis of differentiated (papillary, follicular) thyroid carcinoma ( Memento of March 5, 2009 in the Internet Archive )
- ↑ NA Saamann et al .: The results of various modalities of treatment of well differentiated thyroid carcinoma; a retrospective review of 1599 patients. In: J Clin Endocrinol . tape 75 , 1992, pp. 147-154 . and EL Mazzaferri, SM Jhiang: Long-term impact of initial surgery and medical therapy on papillary and follicular thyroid cancer. In: Am J Med . tape 97 , 1994, pp. 418-428 . quoted from F. Grünwald, C. Menzel. Radioiodine therapy. In: T. Kuwert, F. Grünwald , U. Haberkorn , T. Krause: Nuclear medicine. Stuttgart, New York 2008, ISBN 978-3-13-118504-4 .
- ↑ AM Smith: Carcinoma of the Thyroid. In: Virginia M. Monthly. 69, 1942, pp. 318-324 ( Abstract (PDF) Cancer Res. , 1943; 3, pp. 729-808; accessed December 19, 2009).
- ^ ET Leddy: The Roentgen Ray Treatment of Malignant Tumors. In: M. Clin. North America. 25, 1941, pp. 973-1009 ( Abstract (PDF) Cancer Res. , 1943; 3, pp. 425-496; accessed December 19, 2009).
- ^ C. Bonne: Over Maligne Schildkliergezwellen (Malignant tumors of the thyroid). In: South African M, J. 15, 1941, pp. 147-152 ( Abstract (PDF) Cancer Res. , 1943; 3, pp. 281-352; accessed December 19, 2009).
- ^ Joseph G. Hamilton, Mayo H. Soley: Studies in iodine metabolism of the thyroid gland in situ by the use of radio-iodine in normal subjects and in patients with various types of goiter. In: American Journal of Physiology . 131, 1940, pp. 135-143.
- ^ Joseph G. Hamilton and Mayo H. Soley: Studies in iodine metabolism by the use of a new radioactive isotope of iodine. In: American Journal of Physiology. 127, 1939, pp. 557-572.
- ^ Joseph G. Hamilton, Gordon A. Alles: The physiological action of natural and artificial radioactivity. In: American Journal of Physiology. 125, 1939, pp. 410-413.
- ↑ Joseph G. Hamilton: The rates of absorption of the radioactive isotopes of sodium, potassium, chlorine, bromine, and iodine in normal human subjects. In: American Journal of Physiology. 124, 1938, pp. 667-678.
- ^ S. Hertz, A. Roberts: Application of radioactive iodine in therapy of Graves' disease . In: J Clin Invest . tape 21 , no. 6 , 1942, pp. 624 . quoted from: Martin Metten: The effect of the dose rate on the success of radioiodine therapy in functional thyroid autonomies . DNB 96838837x (dissertation, 2002, Justus Liebig University Giessen).
- ↑ Ralf Paschke, Peter Georgi: Therapy of uni- or multifocal thyroid autonomy. (PDF) In: Dtsch Arztebl. 97, No. 47, 2000, p. A-3197, accessed December 19, 2009.
- ↑ a b L.-A. Hotze. The history of radioiodine therapy. Archived from the original on June 24, 2010 ; accessed on December 31, 2009 .
- ^ AS Keston, RP Ball, VX Frantz, WW Palmer: Storage of radioactive iodine in a metastasis from thyroid carcinoma. In: Science. 95, 1942, pp. 362-363, doi: 10.1126 / science.95.2466.362 ( Abstract (PDF; 793 kB) Cancer Res. , 1943; 3, pp. 281-352; accessed December 19, 2009).
- ↑ HT Wikle, AJ Ritzmann: The course of carcinoma of the thyroid gland. Report of an unusual case. In: Am J Surg 5, No. 6, 1942, pp. 507-512 doi: 10.1016 / S0002-9610 (42) 90721-9 ( Abstract (PDF; 793 kB) Cancer Res. , 1943; 3, p. 281 -352; accessed December 19, 2009).
- ↑ SM Seidlin, LD Marinelli, E. Oshry: Radioactive iodine therapy: effect on functioning metastases of adenocarcinoma of the thyroid. In: JAMA . tape 132 , 1946, pp. 838 .
- ^ E. Siegel: The beginnings of radioiodine therapy of metastatic thyroid carcinoma: a memoir of Samuel M. Seidlin, MD (1895–1955) and his celebrated patient. In: Cancer Biother Radiopharm . tape 14 , 1999, p. 71 .
- ↑ C. Winkler: Radioactive iodine for the treatment of malignant thyroid tumors. In: Verh Dtsch Ges Inn Med . tape 56 , 1950, pp. 180 . quoted from L.-A. Hotze. The history of radioiodine therapy. Archived from the original on June 24, 2010 ; Retrieved February 7, 2012 .