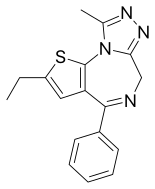
Deschloroetizolam
 | |
 | |
| Clinical data | |
|---|---|
| Dependence liability | Moderate |
| Routes of administration | Oral, sublingual, rectal |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C17H16N4S |
| Molar mass | 308.40 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Deschloroetizolam (also known as Etizolam-2) is a thienotriazolodiazepine that is the dechlorinated analog of the closely related etizolam.[1][2][3] The compound has been sold as a designer drug.[4][5][6][7]
Legal status
Deschloroetizolam is classified and controlled as a hazardous substance in Sweden as of on October 15, 2015.[8]
See also
- Adinazolam
- Clonazolam
- Deschloroclotizolam
- Diclazepam
- Etizolam
- Flubromazepam
- Flubromazolam
- Fluetizolam
- Meclonazepam
- Metizolam
- Nifoxipam
- Pyrazolam
References
- ^ Weber KH, Bauer A, Langbein A, Daniel H (September 1978). "Heteroaromaten mit anellierten Siebenringen, III. Umwandlung von Thienotriazolooxazepinen in Diazepine". Justus Liebigs Annalen der Chemie (in German). 1978 (8): 1257–1265. doi:10.1002/jlac.197819780806.
- ^ US 3904641, Nakanishi M, Tahara T, Araki K, Shiroki M, "Triazolothienodiazepine compounds", published 1975-09-09, assigned to Yoshitomi Pharmaceutical Industries, Ltd.
- ^ EP 0776892, Hiroshi K, Syuji E, Hideaki S, Minoru M, Kenichi O, "Thienylazole compound and thienotriazolodiazepine compound", published 4 June 1997, assigned to Yoshitomi Pharmaceutical Industries, Ltd.
- ^ "Deschloroetizolam". New Synthetic Drugs Database. 9 November 2023.
- ^ Pettersson Bergstrand M, Helander A, Hansson T, Beck O (April 2017). "Detectability of designer benzodiazepines in CEDIA, EMIT II Plus, HEIA, and KIMS II immunochemical screening assays". Drug Testing and Analysis. 9 (4): 640–645. doi:10.1002/dta.2003. PMID 27366870.
- ^ Manchester KR, Maskell PD, Waters L (March 2018). "Experimental versus theoretical log D7.4 , pKa and plasma protein binding values for benzodiazepines appearing as new psychoactive substances". Drug Testing and Analysis. 10 (8): 1258–1269. doi:10.1002/dta.2387. PMID 29582576. S2CID 31098917.
- ^ Manchester KR, Waters L, Haider S, Maskell PD (July 2022). "The blood-to-plasma ratio and predicted GABAA-binding affinity of designer benzodiazepines". Forensic Toxicology. 40 (2): 349–356. doi:10.1007/s11419-022-00616-y. PMC 9715504. PMID 36454409. S2CID 247455284.
- ^ "Nya substanser klassas som narkotika eller hälsofarlig vara" (in Swedish). Folkhälsomyndigheten. 18 August 2015.
