Adamsite- (Y)
| Adamsite- (Y) | |
|---|---|
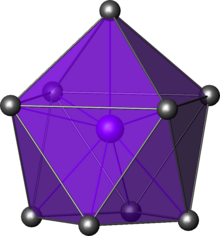
| Radial adamsite (Y) aggregate with orange-red rhodochrosite and shomiokite (Y) . "Poudrette Qarry", Mont Saint-Hilaire , La Vallée-du-Richelieu RCM, Montérégie , Québec , Canada (field of view: 1.3 cm high) | |
| General and classification | |
| other names |
|
| chemical formula |
|
|
Mineral class (and possibly department) |
Carbonates and nitrates |
|
System no. to Strunz and to Dana |
5.CC.30 04/15/08/01 |
| Crystallographic Data | |
| Crystal system | triclinic |
| Crystal class ; symbol | triclinic pinacoidal; 1 |
| Space group | P 1 (No. 2) |
| Lattice parameters |
a = 6.2592 Å ; b = 13.0838 Å; c = 13.2271 Å α = 91.130 °; β = 103.554 °; γ = 90.188 ° |
| Formula units | Z = 4 |
| Frequent crystal faces | {010}, {001} |
| Twinning | often with {001} as the mirror plane |
| Physical Properties | |
| Mohs hardness | 3 |
| Density (g / cm 3 ) | 2.27 (measured); 2.27 (calculated) |
| Cleavage | very perfect after {001}, good after {100} and {010} |
| Break ; Tenacity | not given; brittle |
| colour | colorless to white, occasionally pale pink, rarely pale purple |
| Line color | White |
| transparency | translucent to translucent |
| shine | Glass to mother-of-pearl gloss |
| radioactivity | weakly radioactive |
| Crystal optics | |
| Refractive indices |
n α = 1.480 n β = 1.498 n γ = 1.571 |
| Birefringence | δ = 0.091 |
| Optical character | biaxial positive |
| Axis angle | 2V = 53 ° (measured), 2V = 55 ° (calculated) |
| Pleochroism | unavailable |
| Other properties | |
| Chemical behavior | Rapid dissolution at room temperature with vigorous bubbling in dilute HCl ; crackled in acetone (fast) and ethanol (slow) |
Adamsite- (Y) is a very rare mineral from the mineral class of " carbonates and nitrates ". It crystallizes in the triclinic crystal system with the idealized chemical composition NaY (CO 3 ) 2 · 6H 2 O and is therefore chemically seen a hydrous sodium - yttrium - carbonate .
Adamsite- (Y) mainly forms according to (001) platy and elongated, needle-like to fibrous crystals up to 2.5 cm in length, on which the pinacoids {010} and {001} determine the crop . They mostly come together to form spherical units with a radial structure. Reticulated to mesh-like intergrowths of adamsite (Y) crystals are rare.
The type locality of the adamsite (Y) is the vein- shaped “poudrette pegmatite” opened up by the “poudrette quarry” ( coordinates of the poudrette quarry ) in Mont Saint-Hilaire , regional county community La Vallée-du-Richelieu , Montérégie , Québec , Canada .
Etymology and history

The later called adamsite (Y) mineral was first recognized in 1992 by George Yanji Chao of Carleton University in Ottawa , Canada , as a possibly new mineral species, to which the provisional number "MSH UK-96" was assigned. The material was made available by the collector Gilles Haineault . In 1998 further material was found in the "Poudrette Quarry", but the chemical and X-ray diffraction data of this new find were not comparable with those of the first find, so this material was given the provisional number "MSH UK-106". Another mineral with a similar chemical composition, similar training and from the same environment as adamsite- (Y) was named "MSH UK-91". However, this material is more fine-grained than adamsite (Y) and has a completely different diffractogram than this. Both “MSH UK-96” and “MSH UK-106” later turned out to be identical, “MSH UK-91” is still considered an unnamed Na-Al-REE carbonate. After completing the investigations and determining all relevant data, this mineral was submitted to the International Mineralogical Association (IMA), which recognized it as a new mineral in 1999 under the provisional designation "IMA 1999-020".
The first scientific description of this mineral followed in 2000 by a team of Canadian scientists with Joel D. Grice and Robert A. Gault from the Canadian Museum of Nature (CMN) in Ottawa, Andrew C. Roberts from the Geological Survey of Canada , Ottawa, and Mark A. Cooper from the Department of Geological Sciences, University of Manitoba , Winnipeg , Canada, in the Canadian science magazine The Canadian Mineralogist as adamsite- (Y) ( English Adamsite- (Y) ). They named the mineral after the Canadian mineralogist and petrologist Frank Dawson Adams (1859–1942). Adams was president of the Geological Society of America and the Canadian Mining Institute . He examined the Cretan magmatites of the Monteregian Hills with the Mont Saint-Hilaire and was the first to describe this group of mountains as the " petrographic province of the Monteregian Hills ". The Levinson modifier in the adamsite- (Y) [the suffix "- (Y)"] indicates the dominant rare earth metal (here: yttrium), as the IMA guidelines require when naming minerals containing rare metals .
The type material for adamsite (Y) (cotypes) is kept in the collections of the Canadian Museum of Nature , Ottawa, Canada (catalog numbers CMNMC 82939 and CMNMC 82940), and the Geological Survey of Canada in Ottawa (catalog number NMCC 068086).
classification
Since the adamsite (Y) was only recognized as an independent mineral in 2007 and was first described in 2008, it is not listed in the 8th edition of the mineral classification according to Strunz, which has been out of date since 1977 . In the “Lapis Mineral Directory”, which was last updated in 2018, which is still based on this old form of Karl Hugo Strunz's system , out of consideration for private collectors and institutional collections , the mineral was given the mineral and system no. V / D.03-02 . In the "lapis system" this corresponds to the class of "carbonates, nitrates and borates" and there corresponds to the section "hydrous carbonates, without foreign anions", where adamsite- (Y) together with shomiokit- (Y) , lecoqit- ( Y) , Calkinsit- (Ce) , Lanthanit- (La) , Lanthanit- (Ce) , Lanthanit- (Nd) , Tengerit- (Y) , Kimurait- (Y) , Galgenbergit- (Ce) , Lokkait- (Y) and Hizenit- (Y) forms an independent but unnamed group.
The 9th edition of Strunz's mineral systematics, valid since 2001 and updated by the International Mineralogical Association (IMA) until 2009, assigns the adamsite (Y) to the newly defined class of "carbonates and nitrates", but also to the department of “Carbonates without additional anions; with H 2 O “. However, this is further subdivided according to the relative size of the cations involved and / or the metals primarily involved in the compound, so that the mineral can be found in the sub-section "With rare earth elements (REE)", where it is the only one, depending on its composition Member of the unnamed group with the system no. 5.CC.30 forms.
The systematics of minerals according to Dana , which is mainly used in the English-speaking world , assigns the adamsite (Y) to the common class of "carbonates, nitrates and borates" and there in the department of "hydrous carbonates". Here he is to be found as the only member of the unnamed group 04/15/08 within the subdivision " Hydrogen Carbonates " (without further specifications).
Chemism
Mean values from seven microprobe analyzes on three adamsite (Y) crystals from the type locality yielded 8.64% Na 2 O; 0.05% CaO; 22.88% Y 2 O 3 ; 0.37% Ce 2 O 3 ; 1.41% Nd 2 O 3 ; 1.02% Sm 2 O 3 ; 1.92% Gd 2 O 3 ; 0.56% Tb 2 O 3 ; 3.28% Dy 2 O 3 ; 0.90% Ho 2 O 3 ; 2.83% Er 2 O 3 ; 0.27% Tm 2 O 3 ; 1.04% Yb 2 O 3 ; 25.10% CO 2 ; 29.90% H 2 O (total 100.17%). The presence of H 2 O and CO 2 was confirmed by infrared spectroscopy , and their concentrations were determined by thermogravimetric analyzes . On the basis of 12 oxygen atoms, the empirical formula Na 1.00 (Y 0.72 Dy 0.06 Er 0.05 Gd 0.04 Nd 0.03 Yb 0.02 Sm 0.02 Ho 0 , 02 Ce 0.01 Tb 0.01 Tm 0.01 ) Σ = 0.99 C 2.04 H 11.87 O 12 or simplified Na 1.00 (Y 0.72 Dy 0.06 Er 0.05 Gd 0.04 Nd 0.03 Yb 0.02 Sm 0.02 Ho 0.02 Ce 0.01 Tb 0.01 Tm 0.01 ) Σ = 0.99 (CO 3 ) 2.04 · 5.94H 2 O calculated. This empirical formula can be idealized to NaY (CO 3 ) 2 · 6H 2 O, which contains 9.11% Na 2 O; 33.21% Y 2 O 3 ; 25.89% CO 2 and 31.79% H 2 O (total 100.00%) required. The idealized formula corresponds to the official IMA formula for the adamsite- (Y).
In addition to adamsite- (Y), among the currently known minerals only lecoqite- (Y) , Na 3 Y (CO 3 ) 3 · 6H 2 O, and shomiokite- (Y) , Na 3 Y [CO 3 ] 3 · 3H 2 O, the combination of elements Na – Y – C – O – H; both can be understood as significantly more Na-rich analogues of the Na-poorer adamsite- (Y). Chemically similar are Ashcroftin- (Y) , K 5 Na 5 (Y, Ca) 12 Si 28 O 70 (OH) 2 (CO 3 ) 8 · 8H 2 O, Donnayit- (Y) , NaCaSr 3 Y (CO 3 ) 6 · 3H 2 O, Ewaldite , Ba (Na, Ca, Y, Ce, K) (CO 3 ) 2 · 2.6H 2 O, Mckelveyite- (Y) , NaCaBa 3 Y (CO 3 ) 6 · 3H 2 O , Mineevit- (Y) , Na 25 Ba (Y, Gd, Dy) 2 (CO 3 ) 11 (HCO 3 ) 4 (SO 4 ) 2 F 2 Cl, Peatit- (Y) , Li 4 Na 12 Y 12 ( PO 4 ) 12 (CO 3 ) 4 (F, OH) 8 , Ramikit- (Y) , Li 4 (Na, Ca) 12 Y 6 Zr 6 (PO 4 ) 12 (CO 3 ) 4 O 4 [(OH) , F] 4 , Thomasclarkit- (Y) , (Na, Ce) (Y, REE) (HCO 3 ) (OH) 3 · 4H 2 O, as well as the "UM1990-13-CO: HNaREESrY" not yet described as minerals , Sr 2 Na 2 (Ce, La) Y (CO 3 ) 6 · 3H 2 O?, "UM1992-05-CO: CaCeLaNaSr", (Sr, Na, Y, REE, Ca, Ba) 2 (CO 3 ) 2 · H 2 O, and "Unnamed (MSH UK-37A)", Sr 3 NaCaY (CO 3 ) 6 · 3H 2 O (Sr analogue from Ewaldit).
Crystal structure
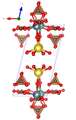
Adamsite- (Y) crystallizes in the triclinic crystal system in the space group P 1 (space group no.2 ) with the lattice parameters a = 6.2592 Å , b = 13.0838 Å, c = 13.2271 Å, α = 91.130 °, β = 103.554 ° and γ = 90.188 ° as well as four formula units per unit cell . Precession single crystal - recordings showed that Adamsit- (Y) by twinning (using {001} as a mirror plane ) a pseudoorthorhombische symmetry in the orthorhombic crystal class dipyramidal- mmm has.
The crystal structure of the adamsite (Y) contains four cation positions with two different polyhedra . The two six-coordinate Na positions are occupied by [Na (H 2 O) 6 ] polyhedra, which can be described as “bifurcated” tetragonal pyramids with the Na atom slightly above the square pyramid base. This base consists of four H 2 O groups, with the “bifurcated” tip (apex) having two additional H 2 O groups. The nine-fold coordination (seven oxygen atoms and two H 2 O groups) around each yttrium position can be described as a tetragonal (square) antiprism with a pyramid on top (" Monocapped Square Antiprism ") with a further ligand is arranged (compare the adjacent figure). All (CO 3 ) polyhedra have edges in common with this (YΦ 9 ) polyhedron, where Φ is an unspecified ligand . Adamsite- (Y) has a layer structure parallel (001). In adamsite (Y) there are thick disks consisting of a unit of Y atoms and parallel, flat lying (CO 3 ) polyhedra, which are sandwiched or parallel between layers of Na (H 2 O) 6 polyhedra and perpendicular to it, "upright" (CO 3 ) polyhedra lies. These [NaY (CO 3 )] disks are separated from one another by [H 2 O] layers. Adjacent [H 2 O] layers are only connected to one another by hydrogen bonds . These weak hydrogen bonds are responsible for the very perfect cleavage of the adamsite (Y) according to {001} and its relatively unstable nature.
| Crystal structure of adamsite- (Y), the blue outline shows the unit cell |
|
|
| Color legend:
__ Y __ Dy __ Gd __ Yb __ Sm __ Ce __ Tb __ Tm __ Na __ C __ O __ H 2 O |
Adamsite- (Y) and Thomasclarkit- (Y) are closely associated with each other at Mont Saint-Hilaire and not only have similarities in chemical composition, but also in common in crystal structure. In both minerals there is a basic unit, which consists of a stacked composite of three polyhedra (each with a C, Y and Na atom as the central atom) with common edges. Although the two sets of polyhedra differ both in the coordination number (Y polyhedron) and in the ligands (OH groups or H 2 O groups in both the Na and Y polyhedra), the basic structural unit is the same . In the adamsite (Y) the Na-YC-tri-polyhedra form planes and have a strong cross-linking in the (001) plane with the (YO 9 ) polyhedra over common corners and edges, which is due to the “flat” carbonate -Groups is reinforced. In the Thomasclarkit- (Y) the Na-YC-tri-polyhedra have common edges and form single chains parallel to [001].
properties
morphology
Adamsite- (Y) is found at its type locality in cavities of a vein-shaped pegmatite and forms there mainly according to [001] and according to (001) platy, needle-like to fibrous crystals up to 2.5 cm in length. On them the second pinacoid {010} and the third pinacoid {001} determine the costume . For a closed crystal a third surface shape is required, which is probably the first pinacoid {100}. The crystals usually come together to form spherical aggregates with a radial structure. Reticulated to mesh-like intergrowths of adamsite (Y) crystals are rare.
physical and chemical properties
The adamsite (Y) crystals are colorless to white, occasionally pale pink, only rarely pale purple. Their line color , however, is always white. The surfaces of the translucent to transparent crystals show a characteristic glassy to mother-of-pearl-like sheen . Adamsite- (Y) has a medium refraction ( n α = 1.480; n β = 1.498; n γ = 1.571) and a high birefringence (δ = 0.091) corresponding to this glass to pearlescent gloss . In transmitted light, the biaxially positive adamsite (Y) is colorless and has no pleochroism .
Adamsite- (Y) has a very perfect cleavability according to {001} and two good cleavages according to {100} and {010}. The mineral is brittle. Adamsite (Y) has a Mohs hardness of 3 and is therefore one of the medium-hard minerals that can be scratched with a copper coin just as easily as the reference mineral calcite (hardness 3). The measured density for adamsite- (Y) is 2.27 g / cm³, the calculated density is also 2.27 g / cm³.
Adamsit- (Y) is not yet in the short wavelength in the long wavelength UV light , a fluorescent . The crystals of the adamsite (Y) dissolve quickly and with strong bubbling at room temperature in dilute hydrochloric acid , HCl. There is rapid crackling in acetone and slow crackling in ethanol .
Precautions
Adamsite- (Y) is classified as weakly radioactive due to its content of rare earth elements and radioactive isotopes of the REE cerium and lanthanum and has a specific activity of about 201 Bq / g (for comparison: natural potassium 30.346 Bq / g). Despite the only weak radioactivity of the mineral, mineral samples of adamsite (Y) should only be kept in dust- and radiation-tight containers, but especially never in living rooms, bedrooms or workrooms. Absorption into the body (incorporation, ingestion ) should also be prevented in any case and, for safety, direct body contact should be avoided and respiratory protection mask and gloves should be worn when handling the mineral .
Education and Locations
Adamsit- (Y) has been known as to its type locality in the transition type "Poudrette pegmatite" (or "Poudrette Dike") found that most discovered in the intrusive, alkaline gabbro syenite complex of Mont Saint-Hilaire Pegmatitkörper forms. Here it is a mineral that was formed late at low temperatures and is found in the cavities of a hydrothermally modified peralkaline pegmatite.
In the mentioned cavities, adamsite- (Y) crystallizes after rhodochrosite and petersenite- ( Ce) , but at the same time with horváthite- (Y) and donnayite- (Y) as well as before Thomasclarkite- (Y), which occurs in epitaxial adhesions on adamsite- (Y ) was observed. The order of formation (succession) is therefore Rhodochrosite ± Petersenite- (Ce) → Adamsite- (Y) ± Horváthite- (Y) ± Donnayite- (Y) → Thomasclarkite- (Y). This crystallization sequence indicates a reduction in carbonate activity and Lewis basicity . A comparison of the formulas of the minerals mentioned shows a reduction in the ratio of the carbonate groups to the number of cations plus the other chemical components involved [(H 2 O), (OH), F]. The decrease in Lewis basicity is influenced by the distribution of the hydrogen atoms. When hydrogen is bound to oxygen, the basicity decreases. In this way, in the transition from adamsite- (Y) to Thomasclarkite- (Y) there is a shift from a carbonate to a bicarbonate. Bicarbonate minerals are typically formed at low temperatures and slightly acidic conditions. Similarly, when transitioning from adamsite- (Y) to Thomasclarkite- (Y), more oxygen atoms are protonated, reducing the strength of the Lewis base from Thomasclarkite- (Y) relative to adamsite- (Y).
Typical accompanying minerals of the adamsite (Y) in the cavities in the "poudrette pegmatite" are aegirine , albite , analcime , ankylite (Ce) , calcite , catapleiite , dawsonite , donnayite (Y), elpidite , epididymite , eudialyte , eudidymite , fluorite , Franconit , Gaidonnayit , galena , Genthelvin , gmelinite , Gonnardite , Horváthit- (Y), Kupletskit , Leifit , microcline , molybdenite , Narsarsukit , natrolite , Nenadkevichit , Petersenit- (Ce), polylithionite , pyrochlore , quartz , rhodochrosite, rutile , Sabinaite , sérandite , siderite , sphalerite , Thomasclarkite (Y), zircon and the not yet described Na REE carbonate "MSH UK-91". Further, also be Shomiokit- (Y) , Rémondit- (Ce) and Niveolanit mentioned as Paragenesis minerals.
As an extremely rare mineral formation, the adamsite (Y) has so far (as of 2019) only been described by five sites. The type locality for adamsite- (Y) is the vein-shaped “Poudrette Dike” or “Poudrette pegmatite” exposed in the “Poudrette Quarry” in the alkaline rock Pluton of Mont Saint-Hilaire , Regional County Community of La Vallée-du-Richelieu , Montérégie , Québec , Canada . The “Poudrette Quarry” also includes the quarries in the former “Demix Quarry”, which were sold to the Poudrette family in 1994 and into which the old “Desourdy Quarry” and “Uni-Mix Quarry” quarries had merged earlier. At the end of 2007, the Poudrette family sold the quarry, the name of which has since been given as “Carrière Mont Saint-Hilaire”. The vein-shaped Poudrette pegmatite is located at the southernmost corner of the new “Poudrette Quarry”, which unites the former “Demix Quarry” in the northwest with the original “Poudrette Quarry” in the southeast to form a single quarry. It is 2–4 m thick, at least 70 m long and was vertically exposed to about 35–40 m. At the time of the first description of the adamsite- (Y), the “Poudrette-Dike” was due on soles 6, 7, 8 and 9 of the “Poudrette Quarry”.
Other sources for adamsite- (Y) include:
- The old, still lying since 1960 and now boozed quarry "Hundholmen" in Tysfjord , Nordland , Norway , one of the deformed Granitpegmatit NYF type (NYF = niobium , yttrium , fluorine edited).
- The small, long-discarded feldspar quartz quarry on Stetind Mountain , the 1,392 m high Norwegian national mountain 135 km northeast of Bodø , Nordland, Norway. The quarry was only in production in the years 1961–1962 and opened up a quartz microcline pegmatite of the NYF type.
- The very large pegmatite body of the agpaitic pegmatite "Shomiokitovoe", discovered in 1996 in the Umbozero mine on Mount Alluaiw , Lowozero massif ( Lowosero tundra , Russian Ловозерские тундры ) in the Murmansk Oblast on the Kola peninsula in northwest Russia . The pegmatite is intensely hydrothermally overprinted, extremely rich in Na and CO 2 and relatively Si, and is known for its very large crystals and many minerals - e.g. B. for up to 20 cm long Shomiokit (Y) crystals.
- The "Papachacra-Pegmatite" near Papachacra in the Belén Department , Catamarca Province in northwest Argentina , stretching over 75 km from Belén , the capital of the department, to Puerta de Corral Quemado .
Sites for adamsite- (Y) from Germany , Austria and Switzerland are therefore unknown.
use
Adamsite- (Y) is only of interest to mineral collectors due to its rarity.
See also
literature
- Joel D. Grice, Robert A. Gault, Andrew C. Roberts, Mark A. Cooper: Adamsite- (Y), a new sodium-yttrium carbonate mineral species from Mont Saint-Hilaire, Quebec . In: The Canadian Mineralogist . tape 38 , no. 6 , 2000, pp. 1457–1466 , doi : 10.2113 / gscanmin.38.6.1457 (English, rruff.info [PDF; 1.9 MB ; accessed on January 7, 2020]).
- Joseph A. Mandarino: New Minerals . In: The Canadian Mineralogist . tape 39 , no. 6 , 2001, p. 1751–1760 , doi : 10.2113 / gscanmin.39.6.1751 (English, rruff.info [PDF; 128 kB ; accessed on March 13, 2019]).
- László Horváth: Mineral Species discovered in Canada and species named after Canadians (The Canadian Mineralogist Special Publication 6) . 1st edition. Mineralogical Association of Canada, Ottawa 2003, ISBN 0-921294-40-9 , pp. 18 .
- Adamsite- (Y) . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 ( handbookofmineralogy.org [PDF; 70 kB ; accessed on January 7, 2020]).
Web links
- Mineral Atlas: Adamsite- (Y) (Wiki)
- Adamsite- (Y). In: mindat.org. Hudson Institute of Mineralogy, accessed January 7, 2020 .
- David Barthelmy: Adamsite- (Y) Mineral Data. In: webmineral.com. Accessed January 7, 2020 (English).
- Adamsite- (Y) search results. In: rruff.info. Database of Raman spectroscopy, X-ray diffraction and chemistry of minerals (RRUFF), accessed on January 7, 2020 .
- American-Mineralogist-Crystal-Structure-Database - Adamsite- (Y). In: rruff.geo.arizona.edu. Accessed January 7, 2020 (English).
Individual evidence
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av Joel D. Grice, Robert A. Gault, Andrew C. Roberts, Mark A. Cooper: Adamsite- (Y), a new sodium-yttrium carbonate mineral species from Mont Saint-Hilaire, Quebec . In: The Canadian Mineralogist . tape 38 , no. 6 , 2000, pp. 1457–1466 , doi : 10.2113 / gscanmin.38.6.1457 (English, rruff.info [PDF; 1.9 MB ; accessed on March 13, 2019]).
- ↑ a b Adamsite- (Y) . In: John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, Monte C. Nichols (Eds.): Handbook of Mineralogy, Mineralogical Society of America . 2001 ( handbookofmineralogy.org [PDF; 70 kB ; accessed on March 13, 2019]).
- ↑ a b Malcolm Back, William D. Birch, Michel Blondieau and others: The New IMA List of Minerals - A Work in Progress - Updated: November 2019. (PDF 1720 kB) In: cnmnc.main.jp. IMA / CNMNC, Marco Pasero, November 2019, accessed January 7, 2020 .
- ↑ a b c d e f Adamsite- (Y). In: mindat.org. Hudson Institute of Mineralogy, accessed January 7, 2020 .
- ^ A b David Barthelmy: Adamsite- (Y) Mineral Data. In: webmineral.com. Accessed January 7, 2020 (English).
- ↑ ALKALI-NUTS, Mont Saint-Hilaire - Mont Saint-Hilaire Unknowns. In: saint-hilaire.ca/msh. Accessed January 7, 2020 (English).
- ^ Frank Dawson Adams: The Monteregian Hills: A Canadian Petrographical Province . In: The Journal of Geology . tape 11 , no. 3 , 1903, pp. 239-282 , doi : 10.1086 / 621075 , JSTOR : 30055562 (English).
- ^ László Horváth: Mineral Species discovered in Canada and species named after Canadians (The Canadian Mineralogist Special Publication 6) . 1st edition. Mineralogical Association of Canada, Ottawa 2003, ISBN 0-921294-40-9 , pp. 18 .
- ↑ Stefan Weiß: The large Lapis mineral directory. All minerals from A - Z and their properties. Status 03/2018 . 7th, completely revised and supplemented edition. Weise, Munich 2018, ISBN 978-3-921656-83-9 .
- ↑ Ernest H. Nickel, Monte C. Nichols: IMA / CNMNC List of Minerals 2009. (PDF 1816 kB) In: cnmnc.main.jp. IMA / CNMNC, January 2009, accessed January 7, 2020 .
- ↑ a b adamsite- (Y). In: Mineralienatlas Lexikon. Stefan Schorn u. a., accessed on January 7, 2020 .
- ^ Joel D. Grice, Robert A. Gault: Thomasclarkite- (Y), a new sodium - rare-earth-element bicarbonate mineral from Mont Saint-Hilaire, Quebec . In: The Canadian Mineralogist . tape 36 , no. 5 , 1998, pp. 1293–1300 (English, rruff.info [PDF; 645 kB ; accessed on March 13, 2019]).
- ↑ Localities for Adamsite- (Y). In: mindat.org. Hudson Institute of Mineralogy, accessed January 7, 2020 .
- ↑ Find location list for adamsite- (Y) in the Mineralienatlas and Mindat (accessed on March 13, 2019)
- ↑ a b Igor V. Pekov, Natalia V. Zubkova, Nikita V. Chukanov, Atali A. Agakhanov, Dmitriy I. Belakovskiy, László Horváth, Yaroslav E. Filinchuk, Elena R. Gobechiya, Dmitriy Yu. Pushcharovsky, Murtazali Kh. Rabadanov: Niveolanite, the first natural beryllium carbonate, a new mineral species from Mont Saint-Hilaire, Quebec, Canada . In: The Canadian Mineralogist . tape 46 , no. 5 , 2008, p. 1343–1354 , doi : 10.3749 / canmin.46.5.1343 (English, rruff.info [PDF; 1,2 MB ; accessed on January 7, 2020]).
- ↑ Description of Poudrette Quarry. In: mindat.org. Hudson Institute of Mineralogy, accessed January 7, 2020 .
- ↑ Description of Hundholmen Pegmatite, Norway. In: mindat.org. Hudson Institute of Mineralogy, accessed January 7, 2020 .
- ↑ Description of Stetinden Pegmatite, Norway. In: mindat.org. Hudson Institute of Mineralogy, accessed January 7, 2020 .
- ↑ Description of Shomiokitovoe Pegmatite, Russia. In: mindat.org. Hudson Institute of Mineralogy, accessed January 7, 2020 .