ozone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Mesomeric boundary structures of the ozone molecule | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | ozone | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | O 3 | ||||||||||||||||||
| Brief description |
colorless to bluish, in high concentration deep blue gas with unpleasantly pungent, chlorine-like "mountain sun smell" |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 48.00 g mol −1 | ||||||||||||||||||
| Physical state |
gaseous |
||||||||||||||||||
| density |
2.154 kg m −3 (0 ° C) |
||||||||||||||||||
| Melting point |
−192.5 ° C |
||||||||||||||||||
| boiling point |
−111.9 ° C |
||||||||||||||||||
| solubility |
very heavy in water (494 ml l −1 at 0 ° C, 570 mg l −1 at 20 ° C) |
||||||||||||||||||
| Dipole moment | |||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
|
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Ozone (from ancient Greek ὄζειν ozein “smell”) is a molecule (O 3 ) made up of three oxygen atoms (O ) and the colorless to bluish gas with a characteristic odor, which consists of a colorless to bluish gas with a high concentration. Ozone molecules in the air break down under normal conditions within a few days to biatomic oxygen (O 2 ) , that is, consisting of two oxygen atoms .
Ozone is a strong and poisonous oxidizing agent , which in humans and animals can irritate the respiratory tract and the eyes as well as promote respiratory diseases. In the troposphere , ozone is the third most powerful driver of global warming (after carbon dioxide and methane ).
The ozone layer in the stratosphere protects living beings on earth from damage caused by high-energy mutagenic ultraviolet radiation from the sun.
history
In the year 1839 , described Christian Friedrich Schönbein , for the first time the unique in the chemical phenomenon that an element in gaseous form in two different molecular forms next to each other constantly - ozone and dioxygen. At first, however, this fact seemed too strange for Schönbein's simple interpretation, an allotropy in the gas state, to have gained general recognition.
The degradation reactions of ozone by nitrogen oxides were first described in 1970 by Paul Josef Crutzen ( Nobel Prize in Chemistry 1995).
Occurrence
The amount of ozone in the atmosphere is given in Dobson units (i.e. per earth's surface) or in ppm (i.e. per substance amount of air). The highest concentration of a few ppm has ozone in the stratosphere . It is created there in the ozone-oxygen cycle . Ozone is harmless in the stratosphere and partially absorbs the sun's ultraviolet radiation . In the air we breathe, however, it is already harmful to health in far lower concentrations; in particular, the locally very different ozone exposure causes irritation of the respiratory tract.
These very different risk assessments in the various atmospheric stratifications very often lead to mix-ups and to underestimating the risks. The health risk of ozone in the air layers close to the ground is due to its reactivity; Ozone is one of the most powerful oxidizing agents .
In clean air areas, the ozone concentration is often higher in summer than in cities. This is because nitrogen oxide (NO) counteracts the formation of ozone. In cities, the NO concentration due to emissions from vehicles (land, water and aircraft) is relatively high. The following reactions take place in detail:
Ozone is created as follows:
At the same time, ozone is broken down again by NO:
If there were not other substances, so-called volatile hydrocarbons or CO , in the lower air layer, no more ozone would be formed, but instead an equilibrium between O 3 , NO and NO 2 is established depending on the solar radiation . The more the sun shines, the more ozone and less NO 2 there is, as the latter is split by UV radiation (reaction 1).
In the (polluted) planetary boundary layer of the atmosphere there are also hydrocarbons that are emitted both by humans ( anthropogenic ) and by vegetation ( biogenic ). They are oxidized by OH radicals , producing peroxide radicals ROO · . These in turn ensure that NO is oxidized to NO 2 without consuming any O 3 , as in reaction 3, i.e.:
When reactions 1 and 2 take place again, net new ozone is formed.
Since NO is emitted by cars and industry, ozone is broken down more quickly in cities (according to Reaction 3) than in rural areas. In addition, in rural areas there are often hydrocarbons that are more easily attacked by OH radicals, which means that reaction 4 takes place more quickly. A well-known example of such an easily degradable biogenic hydrocarbon is isoprene . The exact chain of reactions is described in the article summer smog .
The CFCs (chlorofluorocarbons), which are often mentioned in connection with the ozone layer , are split by UV radiation, creating free chlorine radicals, which in turn can "destroy" many ozone molecules .
education
Ozone is created from ordinary oxygen according to the reaction
-
where Δ H denotes the molar enthalpy of reaction .
Ozone forms in the atmosphere in three main ways:
- High-energy solar radiation splits oxygen molecules in the stratosphere into two individual atoms, which each combine with another oxygen molecule to form ozone. This process of splitting oxygen molecules through high-energy UV-C radiation with a wavelength of <242 nm is known as photodissociation .
- Near the earth, ozone is formed when nitrogen oxides (e.g. NO 2 ) react with oxygen O 2 under the influence of UV radiation . Despite the introduction of the engine catalytic converter , road traffic is indirectly responsible for this form of ozone formation in near-earth layers of air (primarily cities) through the emission of pollutants.
- During thunderstorms : The electrical current flow between the cloud and the ground creates ozone during the lightning discharge (in addition to nitric acid and other substances).
Room air purification devices
When operating room air purification devices, ozone can be formed either deliberately or unintentionally. For example, some ionizers form ozone in order to split and eliminate odor-perceived molecules in the ambient air. However, the breakdown products of nicotine and cigarette smoke, in addition to the ozone itself, harbor high health risks. B. the German Lung Foundation warns against removing the bad smell of smoky rooms with ozone-generating air purifiers. The guideline VDI 6022 Part 5 "Air conditioning, indoor air quality - Avoidance of allergenic pollution - Requirements for the testing and evaluation of technical devices and components influencing the breathing air" therefore recommends determining the ozone emission rate when using ionizers .
Ozone can also in the operation of electrostatic precipitators ( electro-filters ), which are used for indoor air purification arise. This is particularly the case when a negative corona discharge is achieved due to the negative polarity of the spray electrode . This is why this constellation is generally not used in ventilation and air conditioning systems.
Ozone can also arise when operating room air purification devices that specifically generate non-thermal plasma . The amount of ozone generated depends on the design and power consumption of the device used.
Photocopier
With older photocopiers and laser printers , you can smell a typical "ozone smell". This smell is only indirectly due to the ozone formed by the ionization of the air in the device; rather, it comes about through traces of nitrous gases (NO x ), which are formed by the reaction of the ozone with the nitrogen in the air. The functional principle of the devices requires the air to be ionized at voltages of 5–15 kV. Most of the devices have ozone filters that convert the ozone produced into carbon dioxide. However, if possible, these devices should not be used in unventilated rooms. Modern printers and photocopiers work with transfer roller technology , which prevents ozone formation and has largely replaced the older corona wire technology.
Extraction and presentation
Presentation in the laboratory
Ozone can be obtained from the reaction of potassium permanganate with concentrated sulfuric acid. The unstable dimanganese heptoxide Mn 2 O 7 formed as an intermediate product breaks down at room temperature to form manganese dioxide and oxygen , which is rich in ozone.
During the electrolysis of dilute sulfuric acid (approx. 20%), ozone develops on a gold or platinum anode, especially at high current densities. With good cooling, 4–5% ozone content can be achieved in the resulting oxygen, a concentration that is sufficient to be able to carry out all reactions of the ozone on a preparative scale. Using sophisticated equipment (e.g. fine platinum wire coils) and cooling to −14 ° C, significantly higher ozone concentrations can be achieved.
Ozone can also be produced from atmospheric oxygen under the action of ultraviolet radiation or silent electrical discharges. Corresponding devices known as ozonizers are commercially available.
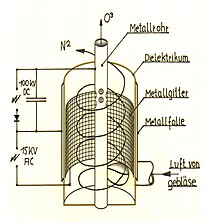
Manufacturing in technology
Because of its instability, ozone cannot be stored for long periods of time or bought in pressurized bottles like other industrial gases. Before it can be used (chemical synthesis, water treatment, as a bleaching agent, etc.), it must be produced on site.
In most cases, dried air or oxygen (dew point at least −65 ° C) is used as a carrier gas for production. In rare cases, oxygen is mixed with argon, carbon dioxide and the like. mixed. In the ozone generator ( ozone generator ), the oxygen molecules are dissociated into oxygen atoms by silent electrical discharge , after which ozone synthesis and ozone enrichment take place in the plasma of the discharge filaments. Typical final concentrations in air are between one and five percent by mass, in oxygen between six and thirteen percent by mass.
From pure, dry oxygen up to 90 g · m −3 , from air (with cooling) up to 40 g · m −3 ozone can be obtained. For 1 kg of ozone from oxygen (in the range of 1–6% by weight) 7–14 kWh of electricity and 1.8 m 3 / h of cooling water are used.
The technical devices used in practice can be based on the following electrode configurations:
- tubes pushed into one another (e.g. glass tube with a metallic inner coating in a steel tube)
- parallel plates
- wire-wound electrodes for surface discharges
- Tip to plate
In systems with more than 20 kg of ozone per hour, only tube ozonizers are usually used.
As a first approximation, ozone enrichment is a function of the electrical energy input per gas volume. The following parameters can be varied to optimize the efficiency:
- Electrode gap
- Electrode alignment
- Dielectric material
- Peak voltage and frequency
By superimposing an inhomogeneous electric field during the energy input ( dielectrophoresis ), the chemical equilibrium, which arises from synthesis and decomposition at a few percent by weight, can be shifted in favor of ozone.
Although ozone is formed from oxygen with heat absorption , ozone generating boilers in industrial applications are water-cooled, as almost 90 percent of the energy introduced has to be removed again due to the high rate of decomposition. The gas temperature is another dominant factor for the efficiency of ozone synthesis.
Because of the high reactivity of ozone, only a few materials are resistant to ozone. These include stainless steel (e.g. 316L), glass , polytetrafluoroethylene (PTFE), perfluoroalkoxy polymers (PFA), polyvinylidene fluoride (PVDF) and perfluorubber . Viton , which must not be exposed to any mechanical alternating stress under ozone, is conditionally resistant .
storage
Liquid ozone can be stored in the form of a 30 to 75% solution in liquid oxygen at −183 ° C in the presence of stabilizers such as CClF 3 , OF 2 , SF 6 or others without the risk of explosion. Gaseous ozone can be stored in its pure state (no contamination by organic compounds, sulfur or certain metals) at −112 to −50 ° C with a slight excess pressure.
properties
Ozone is gaseous under standard conditions. Due to its oxidizing effect, it irritates the respiratory tract in humans and animals. It can even oxidize silver at room temperature. Ozone intake can often lead to severe temple headaches in humans . In high concentrations, the gas has a characteristic pungent-sharp to chlorine-like odor due to the oxidizing effect on the nasal mucous membrane, while in low concentrations it is odorless. The odor threshold is 40 µg / m 3 , but you quickly get used to the odor and then no longer notice it. Pure O 3 is an allotropic form of dioxygen O 2 . At room temperature it exists as an unstable, colorless to bluish, deep blue diamagnetic gas in high concentration , which condenses to a deep blue liquid at −110.5 ° C and solidifies to a black-violet solid at −192.5 ° C (80 K).
The angled polar molecule with a dipole moment of 0.5337 D (corresponds to 1.780 · 10 −30 C · m ) remains in the solid. The OO distance is 128 pm , the angle between the three oxygen atoms is 117 °.
Ozone maintains combustion much more than dioxygen: Many materials flare up at room temperature when they come into contact with pure ozone. Mixtures of pure oxygen and ozone with a volume fraction of 11.5% or more can decompose explosively under atmospheric pressure with a correspondingly high ignition energy. By adding 1% methane or NO 2 , the ignition limit is reduced to approx. 5% ozone.
Ozone is a stronger oxidizing agent than dioxygen and is a very powerful oxidizing agent in acidic solution. The standard electrode potential E ° for the half-reaction
is + 2.07 V. At normal temperatures, ozone oxidizes metals such as silver and mercury to their oxides. It oxidizes halides to halogens , nitrogen oxides to higher nitrogen oxides, sulfur dioxide to sulfur trioxide , iron (II) to iron (III) salts and sulfides to sulfates . It reacts with dry potassium hydroxide to form potassium ozonide . It reacts with organic substances and attacks most types of double bonds in unsaturated compounds such as olefins , cycloolefins , pinenes , aromatics and polybutadienes . It reacts with ethine to form ethinozonide , a cyclic compound with three oxygen atoms.
Ozone easily breaks down to oxygen in the presence of a catalyst such as manganese dioxide or other metal oxides . It also decomposes in the presence of chlorine or bromine. This decomposition also takes place slowly, non-catalytically at normal temperatures and in aqueous solution.
use
Ozone in water treatment
In water treatment , ozone is used, among other things, for the environmentally friendly oxidation of iron, manganese, organic matter and for disinfection. Ozonation is one of the central treatment stages in many drinking water works (see web links).
Surface water can contain higher levels of algae in the warmer seasons. If such water is processed into process water for use in industry, the cleaning effect of the filter systems can be significantly improved by high ozonization . Due to its high oxidation potential, ozone kills both germs and algae to a large extent and improves the filterability of these finely dispersed impurities and thus the cleaning effect.
Ozone is also used in the treatment of municipal and industrial wastewater ( sewage treatment plant ). The ozonation is added after the usual wastewater treatment by microorganisms. However, sewage treatment plants with ozone systems are mostly pilot projects (such as in Regensdorf- Watt in Switzerland), because the production of ozone on such a large scale is expensive, energy-consuming and the protective measures against the toxic and corrosive substance are considerable. It is currently being discussed whether wastewater treatment with the non-toxic activated carbon is not safer, cheaper and more environmentally friendly.
The objectives of further ozone treatment of conventionally treated wastewater are: (a) Killing pathogenic germs ( disinfection ) to protect the receiving water (e.g. with regard to the bathing water directive ) (b) Oxidative elimination / transformation of organic trace substances that are not or only poorly degradable ( especially drug residues).
A disadvantage of ozonation is the creation of unknown and potentially toxic products when ozone reacts with pollutants in the water. The formation of carcinogenic nitrosamines is suspected. Furthermore, some pollutants, for example X - ray contrast media containing iodine , are practically not broken down by ozone. They therefore continue to get into the environment.
Ozone can be used very well in process combinations with downstream biological systems ( biofilters ), for example in the oxidation of the chemical oxygen demand (COD) to the biological oxygen demand (BOD), which is then further processed in the biofilter. Ozone is also used in fish cycles in aquaculture or aquarium systems.
Most of the products or processes named “chlorine-free” use ozone, for example when bleaching paper. In this context “active oxygen” is often used.
Ozone in exhaust gas treatment
In oxidizing gas scrubbing , ozone is used as an oxidizing agent in gas scrubbers to chemically convert substances dissolved in the scrubbing liquid and thus to increase the driving concentration gradient between the gas to be cleaned and the scrubbing liquid. This process is used for inert organic substances and for heterogeneous gas mixtures with often odorous substances. Alternatively, there is the possibility of transferring poorly water-soluble impurities into higher oxidation levels by means of ozone, which is passed into the exhaust gas flow, which can then be removed with a gas scrubber.
Ozone treatment of vehicles
A so-called ozone treatment is carried out in professional vehicle preparation. This can be eliminated in particular in the case of used cars with odor pollution in the interior (e.g. former smoking vehicles). The oxidizing effect of the ozone converts odorous substances into odorless substances. Likewise, germs and odor-causing bacteria are killed - even in otherwise inaccessible places. As a result, the vehicle is disinfected and usually odorless after this treatment.
Washing machines
Some modern washing machines have an ozone program, which uses ambient air and disinfects the laundry and eliminates odors by means of an ozone generator. Laundries also use this technology.
Harmful effects
Ozone in the air we breathe
The EU has long been setting guidelines for ozone concentrations. According to the EU directive, there is no health risk from ozone below 110 µg / m 3 . The population is informed from a one-hour mean value of 180 µg / m 3 , since at this concentration the productivity of sensitive people can be impaired. From around 200 µg / m 3 of ozone, symptoms such as irritation of tears, irritation of the mucous membrane in the throat , throat and bronchial tubes , headaches , increased coughing and deterioration in lung function can occur. Warnings are issued from a one-hour mean value of 360 µg / m 3 , since above this concentration there may be a risk to human health.
In Switzerland, the limit for the one-hour average is 120 µg / m 3 (approx. 60 ppb ). However, this value is exceeded very often. In the hot summer of 2018 , z. For example, in Winterthur the limit value for ozone was exceeded more than 50 times before the end of July, compared to the previous year with 39 times.
A long-term increase in the ozone concentration in the air leads to an increased risk of dying from respiratory diseases . A study published in 2018 shows a link between exposure to ozone and particulate matter and Alzheimer's disease .
Increased immission values occur above all in the area of influence of large industrial areas and highways. Meteorological effects have a strong effect on the local formation and transport of the ozone, so that spatial dependencies can arise over several hundred kilometers.
Concentration increases during heat waves as plants can absorb less ozone. It is estimated that this effect was responsible for 450 additional deaths in the UK, for example, during the 2006 hot summer.
Effects on plants
Ozone has adverse effects on plants and their growth. The concentrations of chlorophyll , carotenoids and carbohydrates decrease , while the aminocyclopropanecarboxylic acid increases and more ethene is formed. It could be shown that increased exposure of citrus plants to ozone triggered protective reactions against oxidative stress . Long-term high levels of ozone pollution can damage deciduous trees, shrubs and crops in particular and reduce their growth, which can lead to yield losses.
Effects on materials
Ozone can damage materials, especially various elastomers and rubbers . In the past, the problem was particularly widespread with tires , but now rarely occurs there due to preventive measures.
Measurement of ozone
Analysis, units
Ozone concentrations used to be and are still predominantly given in ppb (i.e. billionths of a volume, particle or partial pressure fraction) in the USA and are given in µg / m 3 in accordance with the SI . 1 ppb ozone corresponds to 2.15 µg / m 3 (under normal conditions).
Immission measurement
Ozone in the outside air can be recorded photometrically . For this purpose, the continuously sucked in sample air is passed through a measuring cuvette, which is exposed to monochromatic radiation of a certain wavelength. The radiation that passes through and is therefore not absorbed is measured by means of a photodiode or photomultiplier and thus provides information about the ozone concentration in the air. This measurement method is based on the Lambert-Beer law .
Another method for the measurement of ozone in the outside air is the potassium iodide method: in aqueous solution, ozone reacts with potassium iodide, releasing iodine and oxygen. The extinction of the iodine solution is a measure of the ozone concentration in the sample air that has passed through the potassium iodide solution. The process is not selective for ozone. Muenke washing bottles are to be used as absorption vessels.
The differential optical absorption spectroscopy DOAS is also used to measure ozone. Studies for quality assurance of different measurement methods are also available.
The problem with immission measurement of ozone is that no durable test gases can be produced. It is also important to ensure that the materials used cannot react with the ozone.
Bioindication
Effects of ozone can be systematically investigated using tobacco plants . For bioindication , the macroscopically recognizable leaf damage to the plant is used as an action parameter.
literature
- The ozone . In: The Gazebo . Volume 6, 1891, pp. 99 ( full text [ Wikisource ]).
- Dietmar Kunath: Ozone. In: Claus Schaefer, Torsten Schröer (Hrsg.): The large lexicon of aquaristics. Eugen Ulmer, Stuttgart 2004, ISBN 3-8001-7497-9 , p. 734 f.
Web links
As an air pollutant:
- "Ozone is harmful!" (Ozon-info.ch)
- Ozone - A case study (Uni Bielefeld, chemieunterricht.de)
- International Chemical Safety Card (ICSC) for Ozone at the National Institute for Occupational Safety and Health (NIOSH).
Measurement and prediction:
- Ozone . 3-day forecast Europe ( WRF / Chem ), on the website of the Austrian Meteorological Service (ZAMG): Environment: Air quality
- Current information about ozone - ozone values, etc. (Federal Environment Agency)
Individual evidence
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 91st – 100th, improved and greatly expanded edition. Walter de Gruyter, Berlin 1985, ISBN 3-11-007511-3 , p. 535.
- ↑ a b c d e f g h i Entry on ozone in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ W. Schröter, K.-H. Lautenschläger, H. Bibrack: Chemistry. Facts and laws. 16th edition. Verlag Buch und Zeit, Cologne 1992, ISBN 3-8166-0190-1 , p. 226.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 91st – 100th, improved and greatly expanded edition. Walter de Gruyter, Berlin 1985, ISBN 3-11-007511-3 , p. 460.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Dipole Moments, pp. 9-51.
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 10028-15-6 or ozone ), accessed on November 2, 2015.
- ↑ Alfons Ahrens, Michael Becker, Uwe Behmel, Thomas Buscham, Hartmut Evers: Water in the beverage industry . Fachverlag Hans Carl, 2016, ISBN 978-3-418-00912-4 ( limited preview in Google Book Search [accessed September 17, 2019]).
- ↑ spiegel.de August 21, 2020: Ozone levels in the atmosphere are rising
- ↑ Ernst Hermann Riesenfeld : The ozone, its formation and use. In: Die Naturwissenschaften 15, 1927, pp. 777-784, doi: 10.1007 / BF01504659 .
- ↑ a b Renate Eberts: Analysis of the spatial structure of Brandenburg ozone measurement data . ( Memento from September 25, 2015 in the Internet Archive ; PDF) TU Berlin, 1998.
- ^ Ozone in the Atmosphere (accessed May 17, 2008).
- ↑ Federal Office for the Environment (Switzerland): Environmental Series , No. 179.
- ↑ Systemadmin_Environment: Ozone. June 24, 2011, accessed July 5, 2020 .
- ↑ Warning of air purifiers with ozone or ozone generators to remove odors. Federal Association of Pulmonologists , September 10, 2010, accessed on December 8, 2015 .
- ↑ VDI 6022 sheet 5: 2016 11 Room air technology, room air quality; Avoidance of allergenic exposure; Requirements for the testing and evaluation of technical devices and components affecting the breathing air (ventilation and indoor air quality; Avoidance of allergenic exposure; Requirements regarding the testing and evaluation of technical products and components affecting the indoor air). Beuth Verlag, Berlin, p. 21.
- ↑ Hartmut Finger, Ute Schneiderwind, Christof Asbach: Evaluation of mobile room air purification devices. In: Hazardous substances - cleanliness. Air . 75, No. 11/12, 2015, pp. 497–502.
- ↑ VDI 3678 sheet 2: 2010-12 electrostatic precipitator; Process air and indoor air cleaning (Electrostatic precipitators; Process air and indoor air cleaning) . Beuth Verlag, Berlin, p. 11.
- ↑ Henning Heberer, Eberhard Nies, Markus Dietschi, Angela Möller, Wolfgang Pflaumbaum, Marco Steinhausen: Considerations on the effect and toxicological relevance of NTP air purification devices. In: Hazardous substances - cleanliness. Air. 65, No. 10, 2005, pp. 419-424.
- ↑ G. Brauer (Ed.): Handbook of Preparative Inorganic Chemistry. 2nd Edition. vol. 1, Academic Press, 1963, pp. 337-340.
- ↑ Entry on ozone. In: Römpp Online . Georg Thieme Verlag, accessed on June 16, 2014.
- ↑ Google patent search: Patent US3400024 - Inhibiting ozone decomposition with SF 6 , CCl 2 F 2 or CF 4 , accessed July 1, 2018
- ↑ В.Н. Зрелов, Е. П. Серегин: Жидкие ракетные топлива , "Химия", 1975, p. 197
- ↑ Google Patent Search: Patent US3186930 - Method for the production of ozone - Google Patent Search , accessed June 28, 2018
- ↑ Google Patent Search: Patent US3186930 - Method for the production of ozone - Google Patent Search , accessed June 28, 2018
- ^ JG Waller, G. McTurk: Storage of compressed gaseous ozone. In: Journal of Applied Chemistry. 15, 1965, p. 363, doi: 10.1002 / jctb.5010150803 .
- ↑ CS Stokes, WJ Murphy, TR Flint, AE Potter: Storage of Ozone in Dichlorodifluoromethane. In: Industrial & Engineering Chemistry Product Research and Development. 4, 1965, p. 176, doi: 10.1021 / i360015a007 .
- ^ A b Hans Rudolf Christen, Gerd Meyer: Fundamentals of general and inorganic chemistry . Diesterweg, 1997, ISBN 3-7935-5493-7 .
- ↑ Kunihiko Koike, Masaharu Nifuku et al. a .: Explosion properties of highly concentrated ozone gas. In: Journal of Loss Prevention in the Process Industries. 18, 2005, pp. 465-468, doi: 10.1016 / j.jlp.2005.07.020 .
- ↑ Nuclear Research Center Karlsruhe, Institute for Radiochemistry: Results report on research and development work 1984 (PDF)
- ↑ a b Pradyot Patnaik: Handbook of Inorganic Chemicals . P. 684, McGraw-Hill, New York 2002 ISBN 0-07-049439-8
- ↑ Dimitriadou Agapi, Komi Evangelia, Lykou Maria: Urban Metabolism - Water Treatment . in Chapter 5, Section 5.7.
- ↑ VDI 3679 sheet 4: 2014-10 wet separator; Waste gas cleaning by oxidative gas scrubbing (wet separators). Beuth Verlag, Berlin, pp. 3-6.
- ^ Günter Baumbach: Air pollution control. 2nd Edition. Springer-Verlag, Berlin / Heidelberg / New York 1992, ISBN 3-540-55078-X , p. 385.
- ^ Franz Joseph Dreyhaupt (ed.): VDI-Lexikon Umwelttechnik. VDI-Verlag, Düsseldorf 1994, ISBN 3-18-400891-6 , p. 889.
- ↑ VDI 2441: 2016-05 Process gas and exhaust gas cleaning using cold plasma methods; Barrier discharge, corona discharge, UV radiation (Process gas and waste gas cleaning by cold plasma - Barrier discharge, corona discharge, UV radiation). Beuth Verlag, Berlin, p. 12.
- ^ Office for food control and environmental protection of the canton of Schaffhausen: The health significance of the ozone content in the air. Leaflet June 2001.
- ^ Federal Statistical Office & Federal Office for the Environment : Air Quality . (PDF, approx. 6 MB) Chapter 7. In: Environment Switzerland 2007 .
- ↑ What is ozone? → Review at ozon-info.ch .
- ↑ Too high ozone pollution - city reviews action plan. In: landbote.ch , July 19, 2018, accessed on August 2, 2018.
- ↑ Michael Jerrett, Richard T. Burnett et al. a .: Long-Term Ozone Exposure and Mortality. In: New England Journal of Medicine . 360, 2009, pp. 1085-1095, doi: 10.1056 / NEJMoa0803894 .
- ↑ Lilian Calderón-Garcidueñas, Angélica Gónzalez-Maciel and a .: Hallmarks of Alzheimer's disease are evolving relentlessly in Metropolitan Mexico City infants, children and young adults. APOE4 carriers have higher suicide risk and higher odds of reaching NFT stage V at ≤ 40 years of age. In: Environmental Research. 164, 2018, p. 475, doi: 10.1016 / j.envres.2018.03.023 .
- ↑ It's not just the heat - it's the ozone: Study highlights hidden dangers. University of York, accessed January 14, 2014 .
- ↑ Domingo J. Iglesias, Ángeles Calatayuda, Eva Barrenob, Eduardo Primo-Milloa, Manuel Talon: Responses of citrus plants to ozone: leaf biochemistry, antioxidant mechanisms and lipid peroxidation . In: Plant Physiology and Biochemistry . tape 44 , no. 2–3 , 2006, pp. 125-131 , doi : 10.1016 / j.plaphy.2006.03.007 , PMID 16644230 .
- ↑ The ozone levels are falling, but the pollution of the forests remains high. In: wsl.ch. Federal Research Institute for Forests, Snow and Landscape , December 18, 2018, accessed on February 6, 2019 .
- ↑ Madeleine S. Günthardt-Goerg: Ozone symptoms on deciduous trees at selected locations in Eastern Switzerland 2008/2009/2011 . (PDF) In: Ostluft , June 2010, updated February 2013.
- ^ Rising Ozone Levels Pose Challenge to US Soybean Production, Scientists Say. NASA Earth Observatory, July 31, 2003, accessed May 10, 2006 .
- ↑ Randall Mutters: Statewide Potential Crop Yield Losses From Ozone Exposure. (No longer available online.) California Air Resources Board, March 1999, archived from the original on February 17, 2004 ; Retrieved May 10, 2006 .
- ^ Robert W. Layer, Robert P. Lattimer: Protection of Rubber against Ozone. In: Rubber Chemistry and Technology . 63, 1990, p. 426, doi: 10.5254 / 1.3538264 .
- ↑ Fact Sheet ozone. In: Bavarian State Office for the Environment (LfU), Augsburg, lfu.bayern.de. October 2018, accessed November 20, 2019 .
- ↑ DIN EN 14625: 2012-12 outside air; Measurement method for determining the concentration of ozone using ultraviolet photometry; German version EN 14625: 2012. Beuth Verlag, Berlin, p. 11.
- ↑ VDI 2468 sheet 6: 1979-07 measurement of gaseous immissions; Measuring ozone concentration; Direct UV photometric method (basic method). VDI Verlag, Düsseldorf, p. 2.
- ↑ VDI 2468 sheet 1: 1978-05 measurement of gaseous immissions; Measuring ozone and peroxide concentration; Manual photometric method; Potassium iodide method (basic method). VDI Verlag, Düsseldorf, p. 2.
- ^ Franz Joseph Dreyhaupt (ed.): VDI-Lexikon Umwelttechnik. VDI-Verlag, Düsseldorf 1994, ISBN 3-18-400891-6 , p. 663.
- ↑ A. Nawahda: Ozone monitoring using differential optical absorption spectroscopy (DOAS) and UV photometry instruments in Sohar, Oman. I: Environ Monit Assess. 187 (8), Aug 2015, p. 485. PMID 26138853
- ↑ JA Adame, A. Notario, F. Villanueva, J. Albaladejo: Application of cluster analysis to surface ozone, NO 2 and SO 2 daily patterns in an industrial area in Central-Southern Spain measured with a DOAS system. In: Sci Total Environ. 429, Jul 1, 2012, pp. 281-291. PMID 22591990
- ^ EJ Williams, FC Fehsenfeld, BT Jobson, WC Kuster, PD Goldan, J. Stutz, WA McClenny: Comparison of ultraviolet absorbance, chemiluminescence, and DOAS instruments for ambient ozone monitoring. In: Environ Sci Technol. 40 (18), Sep 15, 2006, pp. 5755-5762. PMID 17007137
- ^ Franz Joseph Dreyhaupt (ed.): VDI-Lexikon Umwelttechnik. VDI-Verlag, Düsseldorf 1994, ISBN 3-18-400891-6 , p. 892.
- ↑ Jutta Köhler, Joachim Nittka, Michael Außenendorf, Ludwig Peichl: Long-term observation of immission effects - 30 years of bioindication in Bavaria. In: Hazardous substances - cleanliness. Air . 68, No. 6, 2008, ISSN 0949-8036 , pp. 227-234.
- ↑ VDI 3957 sheet 6: 2003-04 Biological measurement methods for determining and assessing the effects of air pollution on plants (bioindication); Determination and assessment of the phytotoxic effects of ozone and other photo-oxidants; Method of standardized tobacco exposure (Biological measuring techniques for the determination and evaluation of the effects of air pollutants on plants (bioindication); Determination and evaluation of the phytotoxic effect of photooxidants; Method of the standardized tobacco exposure). Beuth Verlag, Berlin, p. 6.